New insights into the mechanism behind the anti-tumor therapeutic effects of Trastuzumab treatment for breast cancer
The overexpression of human epidermal growth factor receptor 2 (Her2/neu) is associated with aggressive breast cancer. Trastuzumab (Herceptin), a humanized monoclonal antibody that binds the extracellular, juxtamembranal domain of HER2, has been used effectively in treating breast cancer in human studies, and can efficiently stop or slow the growth of HER2/neu+ tumors in vitro. Antibody treatment has also been combined effectively with chemotherapeutic agents. The clinical efficacy of trastuzumab has been thought to be due to interference with HER2 signalling and increased susceptibility to chemotherapy-induced apoptosis. However, relapse after the cessation of combined antibody and chemotherapeutic treatments has been reported, and has raised questions about the nature of the therapeutic mechanisms involved and the respective roles of innate and adaptive immunity in these responses.
In a recent paper published in Cancer Cell1, Professors Yangxin Fu, Shengdian Wang and colleagues from the IBP-UC Group for immunotherapy have demonstrated that the therapeutic effect of trastuzumab depends on both innate and adaptive immunity. They demonstrate an interesting antibody-mediated mechanism whereby danger signals are required to mobilize and activate innate cells and prime the adaptive immune system for increased tumor clearance. Park et al show that the activation of innate immunity and T cells is essential for the therapeutic effect of antibody treatment. Importantly, they discovered that while chemotherapeutic drugs administered with antibody treatment can enhance reductions in tumor burden, they can also abolish antibody-initiated immunity and lead to decreased resistance to rechallenge or to earlier relapse. By highlighting this problem, this study should have an important impact on clinical procedures. Park et al provide a strategy that can be used for anti-HER2/neu antibody-mediated tumor clearance.
1. Park SG,Jiang ZJ,Mortenson ED,Deng LF,Radkevich-Brown O,Yang XM,Sattar H,Wang Y,Brown NK,Greene M,Liu Y,Tang J,Wang SD,and Fu YX (2010) The Therapeutic Effect of Anti-HER2/neu Antibody Depends on Both Innate and Adaptive Immunity Cancer Cell 18: 160-170.
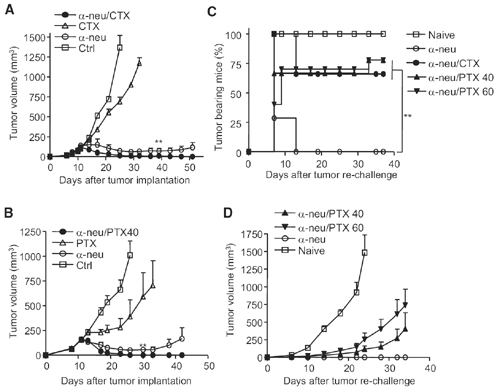
Administration of chemotherapeutics after antibody treatment enhances primary tumor reduction but reduces immunity induced by anti-Neu antibody. WT BALB/c mice (n = 5–10/group) were injecteds.c. with 5 3 105 TUBO cells and treated with100 mg of anti-neu antibody (a-neu) on days 11and 16. Select chemotherapeutic agents wereinjected i.p. at different time points. One of threeexperiments is shown. (A) 100 mg/kg of CTX was injected i.p. on days 16,23, and 33.(B) 40 mg/kg of PTX was injected i.p. on days 14and 19. Treated, tumor-free mice were rechallengedwith 2 3 106 TUBO cells when a primarytumor was not detected for at least 30 days.(C) Percent tumor-bearing mice.(D) Percent mean tumor volume.

