Crystal Structure of the Carnitine Transporter
CaiT is a membrane antiporter that catalyzes the exchange of L-carnitine with γ-butyrobetaine across the E. coli membrane. Prof. Tao Jiang's group recently determined the crystal structure of CaiT to a resolution of 3.15 angstroms. Previous studies have proposed that many membrane transporters operate via an “alternating-access” mechanism, in which the transporter switches between two major alternating conformations, i.e., inward-facing (Ci) and outward-facing (Co). But there is little information on the detailed mechanism underlying precursor/product exchange, especially with respect to the number and structural characteristics of the substrate-binding sites.
The crystal structure of CaiT reveals that CaiT was crystallized as a homotrimer complex, in which each protomer contained 12 transmembrane (TM) helices and four L-carnitine molecules outlining the transport pathway across the membrane. The monomer of CaiT adopt an intermediate state between inward-facing and outward-facing conformation. Mutagenesis studies revealed a primary binding site at the centre of the protein and a secondary substrate-binding site at the bottom of the intercellular vestibule ,which provide mechanistic insights into the association between substrate translocation and the conformational changes of CaiT. This work was published on Nature Structural & Molecular Biology (2010): http://www.nature.com/nsmb/journal/vaop/ncurrent/full/nsmb.1788.html
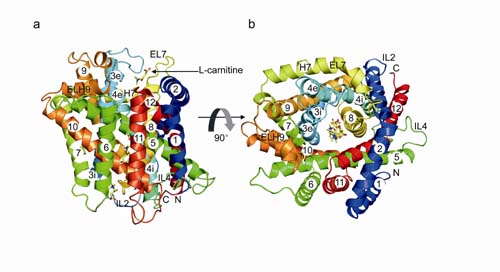
(a) A ribbon diagram of the overall structure of CaiT, viewed parallel to the plane of the membrane. (b) The CaiT structure viewed from the periplasmic side. Four bound l-carnitine molecules are shown using a stick model. All three-dimensional illust…

