New regulatory mechanisms involving MST1 in cell survival signalling
Protein kinases play an important role in the maintenance of homeostasis between cell survival and apoptosis. Deregulation of these kinases leads to various pathological manifestations, such as cancer and neurodegenerative diseases. MST1 (protein kinase mammalian sterile 20-like kinase 1) is a mammalian homologue of Drosophila Hippoand plays a critical role in the regulation of programmed cell death. MST1 exerts its pro-apoptotic function through cleavage, autophosphorylation of its Thr183 residue and subsequent translocation to the nucleus where it phosphorylates a number of molecules, including LATS1/2, FOXO, JNK, and histone H2B.
In two recent papers published in the Journal of Biological Chemistry, Professor Zengqiang Yuan and colleagues report their investigations of the regulation of MST1. They show that the cleavage of MST1 is inhibited by the phosphatidylinositol 3-kinase/Akt pathway, a major cell survival pathway1, and that the stress-activated kinase JNK, a putative substrate of MST, is also an upstream regulator, enhancing MST1 activation by phosphorylating its N-terminal Ser82 residue, providing a feedback regulation loop for MST1 kinase activation2.
Akt is a key upstream regulator of MST1; it interacts with MST1 and phosphorylates the highly conserved Thr120 residue of MST1, which leads to inhibition of its kinase activity and nuclear translocation, as well as to the autophosphorylation of Thr183. Regulation of the MST1 pro-apoptotic function by the PI3K/Akt pathway is via the direct phosphorylation of this highly conserved Thr120 residue.
Aberrant activation of the PI3K/Akt pathway is one of the most common genetic alterations in human malignancy. Elevated levels of pMST1-Thr120 are associated with poor prognosis in ovarian cancer. Professor Yuan and colleagues observed an inverse correlation between pMST1- Thr120 and pMST1-Thr183 in human ovarian tumors, suggesting that it could be a valuable prognostic marker in human ovarian cancer. Inhibition of PI3K/Akt and activation of the pro-apoptotic MST1 kinase may be a novel therapeutic avenue for treatment of human ovarian cancer, since phosphorylation of MST1-Thr120 by PI3K/Akt appears to be a major regulatory mechanism of the Hippo/MST1 pathway in cell survival signaling.
In their second paper, Professors Yuan, Ji and colleagues showed that JNK-induced MST1 phosphorylation promotes caspase-mediated MST1 cleavage, STS-induced MST1 kinase activity, and nuclear translocation as well as dimerization, while inhibition of JNK activation diminishes MST1 cleavage and activity as well as nuclear translocation. They conclude that the JNK-MST1 signaling pathway plays an important role in stress-induced cell death. These research findings suggest that it will be important to explore the role that the JNK-MST1 signaling pathway plays in brain development and oxidative stress-induced neuronal cell death, and to investigate the possibility that JNK and/or MST1 inhibition could be an effective therapeutic approach for related neurological diseases.
1. Zengqiang Yuan, Donghwa Kim, Shaokun Shu, Junbing Wu, Jianping Guo, Lei Xiao, Satoshi Kaneko, Domenico Coppola, and Jin Q. Cheng (2010): Phosphoinositide 3-Kinase/Akt Inhibits MST1-Mediated Pro-apoptotic Signaling through Phosphorylation of Threonine 120. Journal of Biological Chemistry 285 (6): 3815–3824.
2. Wenzhi Bi, Lei Xiao, Yunfeng Jia, Junbing Wu, Qi Xie, Jian Ren, Guangju Ji, and Zengqiang Yuan (2010): c-Jun N-terminal kinase enhances MST1-mediated pro-apoptotic signaling through phosphorylation at Serine 82. Journal of Biological Chemistry 283 (9): 6259-6264.
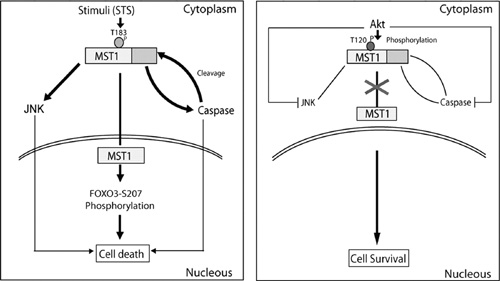
Diagram depicting the regulation of MST1 by Akt. Under stress conditions, MST1 is activated through autophosphorylation of Thr183. The activated MST1 induces the activation of JNK and caspase-3, which in turn enhances MST1 activation through cleavage, and promotes nuclear translocation and cell death. However, Akt phosphorylation of MST1-Thr120 exerts the opposite effect.

