PI4KIIα, a novel player in tumor growth
As a leading cause of death, cancer is an important disease that merits intense investigation. Since the progression of cancer results from the orchestration of various oncogenes and tumor suppressors, the ability to regulate these genes would offer a rational therapeutic approach for cancer treatment. In a recent paper published in Oncogene1, Professor Chang Chen’s lab, in collaboration with Professor Zhihai Qin, reports a new regulator of tumor growth, phosphatidylinositol 4-kinase type IIα (PI4KIIα), and the mechanism of its action in angiogenesis and HIF-1α regulation.
Results from a human cancer tissue microarray showed that PI4KIIα protein expression increases markedly in seven different types of cancers. Suppression of PI4KIIα led to retarded tumor growth in nude mice, and down-regulation of PI4KIIα in cancer cells eliminated tumor cell-induced endothelial cell tubulogenesis and migration, resulting in impaired angiogenesis. Further investigation showed that PI4KIIα can directly regulate HIF-1α expression and that the expression of these two proteins is correlated in vivo. Down-regulation of PI4KIIα also markedly reduced HER-2 autophosphorylation, and PI4KIIα specifically triggered HIF-1α accumulation through a PI3K and ERK-dependent pathway, suggesting that PI4KIIα may regulate HIF-1α through the HER-2/PI3K, ERK cascade.
In summary, Professor Chen’s group discovered a pivotal role for PI4KIIα in the regulation of tumor growth and their results shed new light on the function of PI4KIIα in cancer. In addition, since PI4KIIα is just one subtype of the PI4K family, specific downregulation of PI4KIIα should not induce complicated side effects because other PI4K family members remain functional. Therefore, PI4KIIα may be a promising target for tumor therapy.
1 Jiangmei Li, Yu Lu, Jinhua Zhang, Zhihai Qin, Chang Chen (2010). Phosphatidyliniositol 4-Kinase Type IIα (PI4KIIα) is Crucial for Upregulation of Hypoxia-inducible Factor-1α (HIF-1α) and Tumor-induced Angiogenesis. Oncogene 29: 2550-2559.
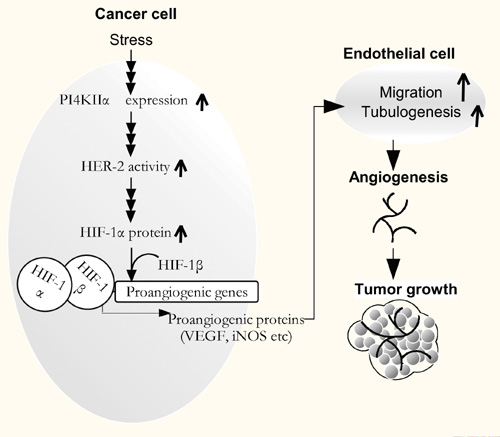
A model for the mechanism of PI4KIIα action in tumor growth. Environmental stress causes an increase in PI4KIIα protein levels, and the elevated PI4KIIα leads to increased HER-2 activities, which can increase HIF-1α protein accumulation in the PI3K- and ERK1/2- dependent pathways. The accumulated HIF-1α protein heterodimerizes with HIF-1b to activate the transcription of downstream proangiogenic genes, such as VEGF and iNOS, which can promote endothelial cell migration and tubulogenesis. As a result, tumor growth is promoted by the enhanced angiogenesis. Knockdown of PI4KIIα significantly inhibits this pathway and represses tumor growth.
Interview with Professor Chang Chen
By Joy Fleming July, 2010
1. What is the research focus of your laboratory? We’re looking mainly at two areas. Firstly, at the regulatory mechanisms of redox reactions. We’re looking for novel proteins and genes involved in free radical generation. Secondly, we’re investigating the mechanisms by which free-radicals and natural products, including antioxidants and Chinese medicinal compounds, interact with proteins and play functional roles in pathological diseases.
2. What are the main breakthroughs/achievements your research group has made in the last three years?
i.) We made the S-nitros(yl)ation detection assay more simple, direct, quantitative and specific.
ii.) We have proposed a new concept: that NO bioactivity is not only controlled by its synthesis, but also by its metabolism (i.e. that NOS(NO synthase)-GSNOR(S-nitrosoglutathione reductase) double-gate control should be considered). This implies that GSNOR could be a good target for NO regulation.
iii.) We discovered new functions of S-nitrosylation in the DNA repair system, in nuclear transport and in the regulation of sumoylation.
iv.) We discovered that hypoxia induces the expression of PIK4IIa, and that PIK4IIa plays a role in cancer.
3. Where does this paper fit into the story of the work your lab is doing? We are interested in the mechanisms underlying hypoxia. Once we discovered that PIK4IIa is induced in hypoxia, we decided to determine its function. Hypoxia is related to a lot of diseases, and is a key factor in the onset of cancer and metabolic disorders. We already had a background in PI4K research as we did a project several years ago on PI4K, screening for natural compounds that can regulate it. We found some natural products that can inhibit and some that can elevate its activity.
4. You’ve recently published your research in Oncogene[JF1] . What were the major findings in your paper?
i) We found that PI4KIIa plays a critical role in cancer. PI4K was previously thought just to be a substrate for PI3K. This is the first time that PI4KIIa has been shown to to be involved in the induction of cancer in its own right.
ii) We found that the mechanism of PI4KIIa action is via HIF 1a and angiogenesis.
5. Can you summarise the significance of your paper in layman’s terms? The discovery that PI4KIIa is involved in the induction of cancer opens up the opportunity to use it as a target for drug discovery, since inhibiting its enzyme activity may suppress tumor growth. We investigated 20 types of cancer and found that levels of PI4KIIa were generally increased compared to normal tissue. This was particularly evident in seven types of cancer, including breast cancer. We used gene-silencing techniques (siRNA) to inhibit the expression of PI4KIIa and found that this caused significant reduction in tumor growth, particularly in breast cancer.
6. What are the next steps in this work and what’s your strategy? There are a number of steps leading from this work:
i.) We will now focus specifically on breast cancer to understand the effects of inhibiting PI4KIIa on tumor metastasis and prognosis.
ii.) We will investigate the molecular mechanism of how PI4KIIa regulates HIF1a. We showed in this paper that phosphorylation of Her2 is involved, but there are still many gaps in the mechanism that we need to fill in.
iii.) We want to see whether PI4K has important functions in diseases of the neurosystem or metabolic disorders. We know it is involved in protein trafficking and may affect neuronal signal transduction. Understanding the function that PI4K plays in metabolic regulation will provide clues to the links between metabolic regulation and associated disorders.
7. Where do you see your research leading in the future? Our current research on PI4K is basic research on its transcriptional regulation, interacting proteins and specific inhibitors. We believe the information generated from our research will contribute to the field, leading to the discovery of its role and development of therapeutic applications.
In our research on thiol modifications we want to study the dynamic changes and functions of these modifications in disease progress. We also want to explore how thiol modification may affect other types of post-translational modifications.
8. What are the implications of your research for society? Oxidative stress is an important factor in the onset of disease. Thiol modification plays an important role in oxidative stress. Through our work we hope to demonstrate the mechanism underlying the relationship between thiol modification and oxidative stress. This will be of great value for understanding and treating oxidative-stress related diseases.
Our work on PI4KIIa will help provide an understanding of its role in disease, and possibly lead to new therapeutic strategies for the treatment of some kinds of cancer.
9. What else is your lab working on now? We’re investigating free-radical homeostasis (ie the biology of the generation and function of free radicals). Our knowledge of the proteins involved in the generation of free radicals hasn’t changed much in the last 50 years. But we have new high-throughput techniques now that can be used to identify other novel proteins that are involved.
We’re also looking for key proteins that are modified by redox reactions and investigating the functional significance of these modifications.
We’re looking at the role of PI4K in other diseases and are currently constructing transgenic mice to investigate this.
10. How did you get into this area of research? My PhD research was on the effects of radiation. Of course, one of the major effects of radiation is the generation of free-radicals. So, it was really through my PhD research that I got interested in free radicals and their effects on cells.
11. Which of your professional achievements brings you the most satisfaction? Our work on S-nitros(yl)ation - both method development and functional studies. We’ve developed an efficient and specific method for detecting S-nitros(yl)ation that is well-suited to conducting functional studies of this modification. Using our new method we’ve found new functional modifications involved in the regulation of protein translocation, stability and neuronal toxicity.
Our discovery that PI4KIIa is an oncogene has also been recognized by the wider scientific community.
12. What are the big issues/outstanding problems that have to be addressed in your field? In free-radical biology, in spite of 50+ years of research, we still do not know how free radicals are generated. Only a few of the proteins involved in free-radical generation have been identified. This is just a small part of the whole story. The physiological roles of free radicals are also unknown. It’s likely that they are signaling molecules. The only knowledge that we have is on the effects of over-production of free radicals – i.e. their impact on the development of disease.

