Teams led by Rao Zihe and Hu Junjie make progress in mitochondrial inner membrane fusion research
On May 7, 2020, a research paper titled ‘Structural insights into G domain dimerization and pathogenic mutation of OPA1’, which was jointly written by teams led by CAS Academician Rao Zihe and by Hu Junjie, was published online by the Journal of Cell Biology. This research studied the crystal structure of minimal GTPase domain (MGD) of mitochondrial endomembrane fusion protein OPA1 in the presence of GDP and BeF3?, and elaborates the molecular basis of GTPase OPA1 dimerization and pathogenesis.
Mitochondria are double membrane–bound organelles that frequently fuse and divide. These membrane dynamics play important physiological roles, including mitochondrial genome maintenance. The fusion of mammalian inner mitochondrial membranes (IMMs) is mediated by dynamin-like GTPase OPA1. Cells lacking OPA1 exhibit loss of mitochondrion DNA. Deletion of OPA1 in mice is lethal, and mutations in human OPA1 cause optic atrophy. Rao and Hu’s teams have previously collaborated on researching the mechanism of Mitofusin (MFN)-mediated membrane tethering and fusion, and earlier this year, on the structure of yeast homologue Mgm1 and a preliminary model of mitochondrial inner membrane fusion. Recently, Sun Fei’s team from the National Laboratory of Biomacromolecules, CAS, studied cryo-EM structures of S-OPA1 to reveal its interactions with membrane and changes upon nucleotide binding (https://doi.org/10.7554/eLife.50294). However, OPA1 remains the only member in the Dynamin superfamily that does not yet have high-resolution structure.
Rao and Yan Liming (PhD) led their teams in determining the crystal structure of the minimal GTPase domain (MGD) of human OPA1. They found that similar helical arrangements were seen in the dynamin-1 GTPase and GED (GG) dimer and the short form Mgm1 (s-Mgm1) structure. Hu Junjie’s team used bio-chemical experiments such as enzyme activity assay, in-vitro polymer analysis, and membrane association as well as morphological replenishment experiments on mitochondria in cells to verify the structural findings. In addition, it has been identified that the N-terminal of OPA1-MGD is followed by a helix bundle (HB) domain and the N-terminal extension mediates nucleotide-independent dimerization. Both forms of dimerization in OPA1 play vital roles in maintaining mitochondrial morphology.
CAS Researcher Hu Junjie is the correspondence author, and Zhao Jinghua (from Hu Junjie’s project team), Yu Caiting (from Rao Zihe’s project team) and Yan Liming are co-first authors. This research was supported by the National Key Research and Development Program, the National Natural Science Foundation of China, and the Strategic Priority Research Program of the Chinese Academy of Sciences (B).
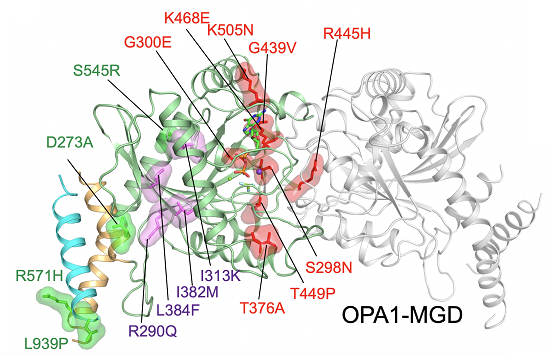
Crystal Structure of OPA1-MGD and Disease-causing Mutations in OPA1
Article Link: https://doi.org/10.1083/jcb.201907098
(Reported by Dr. Hu Junjie's group)

