A novel electron-transport and proton-translocation coupling model revealed by the cryo-EM structure of the photosynthetic alternative complex III
On July 29, 2020, a new research from the group of SUN Fei at Institute of Biophysics and the groups of XU Xiaoling and XIN Yueyong at Hangzhou Normal university was published on Science Advances, entitled "Cryo-EM structures of the air-oxidized and dithionite-reduced photosynthetic alternative complex III from Roseiflexus castenholzii". The researchers revealed the first high-resolution structures of alternative complex III (ACIII) from Roseiflexus castenholzii at both oxidized and reduced states, and proposed a novel coupling mechanism between the electron-transfer and proton-translocation based on structural comparisons and analyses.
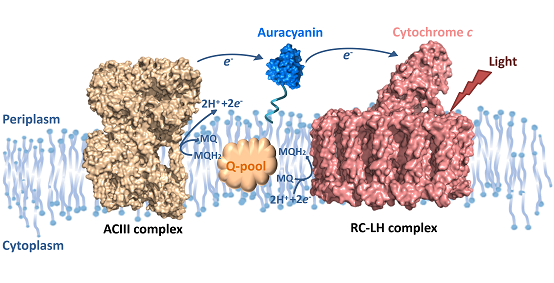
Figure 1. The cyclic electron transfer chain in Roseiflexus castenholzii.
Roseiflexus castenholzii is a thermophilic anoxygenic photosynthetic bacterium, it forms a unique and high-efficient solar energy harvesting, transformation, and cyclic electron transfer system during evolution. It does not contain the antenna complexes. The only light harvesting complex (LH) is assembled by several LHαβ subunits to form a ring like structure, having the L, M and cytochrome c subunits composed reaction center (RC) tightly bound in middle. Each LHαβ contains bacterial chlorophyll and carotenoids to absorb and transfer the light. Once the special BChl pair in RC receive the energy from LHs, the charge separations occur and the menaquinone is reduced to hydroquinone by accepting the resulting electrons. The reduced hydroquinone then diffuses from its binding site to the membrane pool through a gap in the LH ring. Different from higher plants or cyanobacteria, the hydroquinone is oxidized by a novel complex named alternative complex III (ACIII), instead of cyt bc1/b6f complex. ACIII catalyzes the oxidation of menaquinol and mediates the released electrons transferring to a periplasmic blue copper protein auracyanin, which in turn completes a cyclic electron transfer back to the RC (Figure 1). The quinol oxidation is coupled with the transmembrane proton translocation to build up a transmembrane proton gradient, which drives reduction of NADP+ and the formation of ATP that is required for bacterial growth.
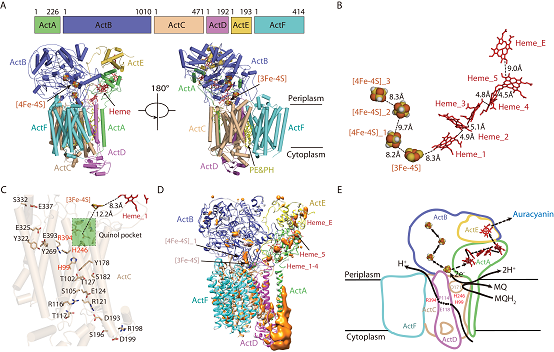
Figure 2. The structure and electron transfer mechanism of ACIII in Roseiflexus castenholzii.
As a functional counterpart for the cyt bc1/b6f complex, the fundamental coupling mechanisms underlying the menaquinol oxidation and proton translocation of the respiratory and photosynthetic ACIII is yet unclear for a long time. In this work, the researchers determined the cryo-electron microscopic (cryoEM) structures of ACIII in the air-oxidized and dithionite-reduced state at 3.3 ? and 3.5 ? respectively. ACIII was comprised of six subunits (ActA, B, C, D, E, and F) that form 23 transmembrane helices. On the periplasmic side, one [3Fe-4S] and three [4Fe-4S] clusters in ActB and six c-type hemes (five in ActA and one in ActE) constitute the electron transport system in ACIII (Figure 2) .
Based on sequence alignments and structural comparisons, the researchers found a menaquinol binding pocket and a proton translocation passage in ActC. Three strictly conserved residues (His99, His246 and Arg394) in the middle passage form extensive hydrogen bonding interactions with residues in the menaquinol binding pocket, which was supposed to play a key role in coupling menaquinol oxidation and proton translocation. Based on the difference map between the oxidized and reduced state as well as the redox potentials of electron carriers, they proposed a novel coupling mechanism between electron-transfer and proton-translocation (Figure 3), which provides a new conceptual insight for the studies of other similar electron transfer-proton translocation coupled phenomenon in the photosynthesis and respiration system.
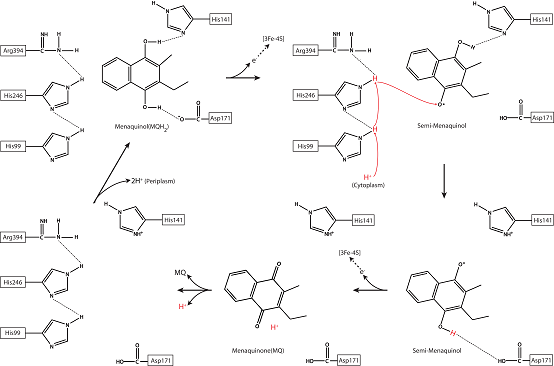
Figure 3. Proposed redox-coupled proton translocation mechanism of the photosynthetic ACIII.
(Image by Dr. SUN Fei’s group)
Prof. XU Xiaoling from Hangzhou Normal University and Prof. SUN Fei are the co-corresponding authors of this work. Dr. SHI Yang from Prof. SUN Fei's group (now working as a post-doc in MRC-LMB based on the UK), associate professor XIN Yueyong from Hangzhou Normal University and WANG Chao, a master student in Prof. XU Xiaoling's group, are the co-first authors. This work was funded by the National Natural Science Foundation of China, Chinese Academy of Sciences, Ministry of Science and Technology of China and the Zhejiang Provincial Natural Science Foundation. The cryoEM works were performed at the Center for Biological Imaging (CBI), Institute of Biophysics, Chinese Academy of Sciences and Center of Cryo-Electron Microscopy (CCEM), Zhejiang University.
This work extends the team's work on RC-LH from Roseiflexus castenholzii. (Nature communications, 9 : 1568. doi: 10.1038/s41467-018-03881-x)
The web link for this paper is https://advances.sciencemag.org/content/6/31/eaba2739
Contact: SUN Fei
Institute of Biophysices, Chinese Academy of Sciences
Beijing 100101, China
Email: feisun@ibp.ac.cn
(Reported by Dr. SUN Fei's group)

