Nanozyme-activated prodrug strategy for targeted tumor catalytic therapy
Enzyme prodrug therapy which was introduced in 1985 is an attractive strategy for targeted cancer therapy with low systemic toxicity. The prodrug itself is low-toxic or non-toxic, after it was ingested, the enzyme can selectively activate the prodrug at the site of the lesion, thus enhancing the pharmacological effect and reducing the side effects. Due to the deficiency of endogenous enzyme activity and its wide distribution in normal tissues, exogenous enzyme prodrug therapy strategy had attracted researchers' attention. Among them, horseradish peroxidase (HRP) and indole-3-acetic acid (IAA) have been demonstrated as an effective enzyme prodrug system. HRP can catalyze the prodrug IAA to produce highly toxic free radical products and kill tumors effectively, so it has a broad application prospect in tumor treatment. HRP can be selectively introduced into tumor cells through antibody conjugation or gene delivery. However, conjugated antibody targeting is limited by missed target in vivo and acting outside tumor cells so as to limit its tumor killing effect. At the same time, the selective expression of exogenous HRP in tumor cells is difficult, and transgenic safety is also a problem. Therefore, a peroxidase mimic enzyme with tumor targeting and enzyme activity is needed.
Since our first report of peroxidase-like activity of Fe3O4 nanoparticles in 2007, nanozymes have grown into a superfamily of artificial enzymes. Due to the advantages of robust catalytic activity, high stability, simple preparation and low-cost, nanozymes have exhibited great potentials in biosensors, environmental protection and anti-biofouling. Recently, significant progresses have been made in applying nanozymes for disease diagnosis and as therapeutics in vivo. As many of the nanozymes are made from metal or metal oxide and susceptible to destabilizing oxidation under acidic conditions, their in vivo applications are limited and often complicated by the potential metal toxicity and long-term biosafety. Thus, the metal-free nanozymes that possess robust activities are desirable to achieve effective in vivo theranostic applications.
Here in, under the guidance of theoretical predictions, we designed and fabricated a novel phosphorous (P) and nitrogen (N) dual-doped porous hollow carbon sphere nanozyme (PNCNzyme) that exhibitsa significantly improved peroxidase-like activity as compared to single N doped counterparts. A prodrug activation strategy for tumor catalytic therapy was then developed based on the as-prepared metal-free nanozyme. It has been shown that IAAcould be effectively loaded onto PNCNzymes to form PNCNzymes@IAA nanoparticle via π-π interactions, which does not affect the enzymatic activity of PNCNzymes. Moreover, the modification of FA ensures the tumor targeting and effective endocytosis of PNCNzymes@IAA nanoparticles in tumor cells. Once located in lysosomes under acidic conditions, the peroxidase activity of PNCNzyme effectively activate the prodrug IAA to produce abundant free radical intermediates, which subsequently trigger reactive oxygen species (ROS) storm and ultimately leads to apoptosis of cells through the mitochondrial pathway (Scheme 1). In addition, the formulations of PNCNzyme-IAAs system exhibited excellent biosafety in vivo. Thus, the strategy achieves delivering prodrugs, targeting tumor cells and activation of prodrugs in one nanoparticle.Taken together, this rationally designed nanozyme based-strategy achieves efficient activation of prodrug selectively in tumor sites, and may inspire new approaches for tumor catalytic therapy.
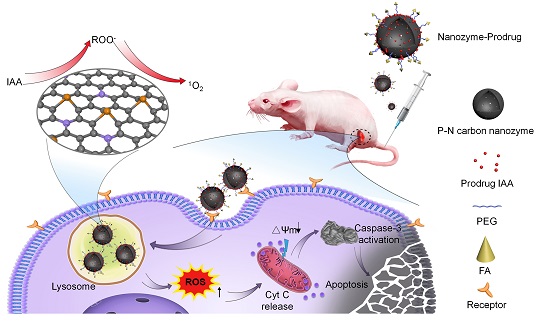
Scheme 1 Schematic illustration of the metal-free nanozyme-activated prodrug strategy for targeted tumor catalytic therapy
(Image by Dr. FAN Kelong’s group)
Prof. YAN Xiyun, Prof. GAO Lizeng and Prof. FAN Kelong are the co-corresponding authors. LIANG Qian, Dr. XI Juqun and Dr. GAO Xuejiao are the co-first authors. The project was funded bythe Strategic Priority Research Program of CAS, National Natural Science Foundation of China, the Key Research Program of Frontier Sciences of CAS and the Youth Innovation Promotion Association of the CAS.
The web link of this paper: https://www.sciencedirect.com/science/article/pii/S1748013220301043
Contact: FAN Kelong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: fankelong@ibp.ac.cn
(Reported by Dr. FAN Kelong’s group)

