WANG Xiaoqun's Group reveals the cellular and molecular properties of neural progenitors in the developing mammalian hypothalamus
The hypothalamus is the central regulator of vital physiological homeostasis and behavior in mammalian. This complex brain region exhibits highly conservation across mammals. In contrast to the cortex that is organized in a columnar structure, hypothalamic neurons form multiple functional nuclei arrayed in a three-dimensional structure to regulate behavior. The organizational principles of cortical lamination have been well studied in the past ten years. The previous study of Wang Xiaoqun’s research group revealed that both of radial glial cells (RG) and outer raial glial cells (oRG) cells are primary progenitor cells during cortical development (Nature Neuroscience 2011). Recent works has made progress to profile the molecular features of distinct cortical progenitors and their contribution to evolutionary expansion of cerebral cortex in (Cell Stem Cell 2017, Nature 2018). However, important aspects of hypothalamus development remain unaddressed, particularly the cellular and molecular properties of hypothalamic neural progenitors involving in hypothalamic neurogenesis.
A research entitled “Cellular and molecular properties of neural progenitors in the developing mammalian hypothalamus” was published online in the journal Nature Communications on Aug 13th, 2020. It describes the developmental characteristics of hypothalamic neural progenitor.
In this research, hypothalamic radial glial (hRG) and hypothalamic mantle zone radial glial (hmRG) cells were found to be neural progenitors in the developing mammalian hypothalamus. The hmRG cells originated from hRG cells and produce neurons. During the early development of hypothalamus, neurogenesis occured in radial columns and is initiated from hRG cells. The radial glial fibers were oriented toward the locations of hypothalamic subregions which acted as a scaffold for neuronal migration.
Using the single-cell RNA sequencing, the researchers revealed progenitor subtypes in human developing hypothalamus and characterized specific progenitor genes. They demonstrated that HMGA2 was involved in E2F1 pathway, regulating the proliferation of progenitor cells. Different neuronal subtypes started to differentiate and expressed specific genes of hypothalamic nucleus at gestational week 10. Moreover, researchers revealed the developmental conservation of nuclear structures and marker genes in mouse and human hypothalamus. These data provided a basic understanding of neurogenesis and regional formation of the non-laminated hypothalamus.
Dr. WANG Xiaoqun is the corresponding author. Dr. ZHOU Xin is the first author. This work was supported by grants from the National Basic Research Program of China, Strategic Priority Research Program of the Chinese Academy of Sciences, the National Natural Science Foundation of China (NSFC) and Beijing Brain Initiative of Beijing Municipal Science & Technology Commission.
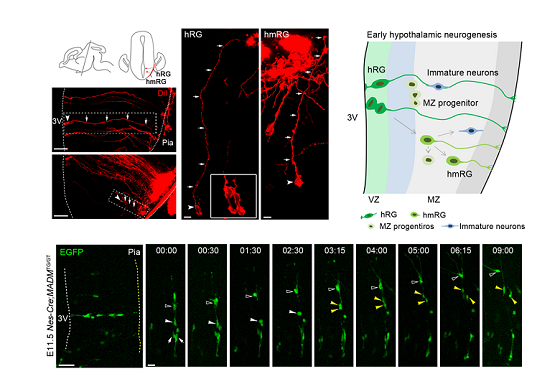
Progenitor composition in the developing mammalian hypothalamus.
(Image by Dr. WANG Xiaoqun's group)
Reference
1. Wang X, Tsai JW, Lamonica B,Kriegstein AR. A new subtype of cortical progenitor cell in the mouse embryonic neocortex. Nat Neurosci. 2011, 14, 555–561.
2. Zhong S, Zhang S, Fan X, Wu Q, Yan L, Dong J, Zhang H, Li L, Sun L, Pan N, Xu X, Tang F, Zhang J, Qiao J, Wang X. A single-cell RNA-seq survey of the developmental landscape of the human prefrontal cortex. Nature. 2018 Mar 22;555(7697):524-528.
3. Liu J, Liu W, Yang L, Wu Q, Zhang H, Fang A, Li L, Xu X, Sun L, Zhang J, Tang F, Wang X. The Primate-Specific Gene TMEM14B Marks Outer Radial Glia Cells and Promotes Cortical Expansion and Folding, Cell Stem Cell. 2017 Nov 2;21(5):635-649.e8. doi: 10.1016/j.stem.2017.08.013.
The web link of this paper: https://www.nature.com/articles/s41467-020-17890-2
Contact: WANG Xiaoqun
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: xiaoqunwang@ibp.ac.cn
(Reported by Dr. WANG Xiaoqun's group)

