Research Teams Cooperate to Uncover Invasion Mechanism and Neutralizing Mechanism of EV-B Species
Enterovirus is one of the most widespread viruses in humans. The outbreak of enterovirus, which can be grouped into different families with various serotypes, has taken place in many countries and regions. The injection of vaccines will create herd immunity effect for susceptible population and inhibit the spread of a serotype-specific when it breaks out in some areas. However, other serotypes of enterovirus are expected to come into being at the same time and they will grow into leading etiological agents of some diseases in a specific group of people.
The changes of enterovirus spectrum have reminded us that we shall have a thorough understanding of echovirus and other typical serotypes and make adequate preparation for the outbreak of any potential epidemic. Echovirus 30 (E30) is an important member of the human enterovirus-B (EV-B) species and has emerged as one of major causative agents of viral encephalitis and meningitis. E30 has broken out seasonally and periodically in Europe, Asia and South America in recent years. However, we still know very little about the antigenic features, immune characteristics and invasion mechanism of enterovirus-B species. In addition, there are no approved vaccines or antiviral therapies available for the treatment of infections caused by EV-B species.
The Nature Communications journal on September 4 published a pair of companion papers, titled "Structures of Echovirus 30 in complex with its receptors inform a rational prediction for enterovirus receptor usage" and "Serotype specific epitopes identified by neutralizing antibodies underpin immunogenic differences in Enterovirus B subtypes". The papers are co-authored by the research teams of Prof. WANG Xiangxi and Academician RAO Zihe, both of whom are from the Institute of Biophysics (IBP) at the Chinese Academy of Sciences (CAS), in collaboration with the research team of Researcher ZHU Fengcai with Jiangsu Provincial Center for Disease Control and Prevention. Participants conducted in-depth analyses of the atomic structure, cellular invasion mechanism and neutralization mechanism of E30, laying a foundation for the prediction of receptor usage and immune features of enterovirus as well as the research and development of relevant drugs and vaccines. It was another important achievement made by them after they had completed the research of EV71, CVA16 and CVA10 in terms of their whole-particle structure and relevant functions in different life stage.
During their research, participants utilized the technology of cryo-electron microscopy to capture and decipher the atomic structure of E30 particles in different life stages, which included young E-particles, uncoating A-particles and mature F-particles reconstructed to 2.9 angstroms, 2.9 angstroms and 3.5 angstroms on the basis of the gold-standard Fourier shell correlations (FSC). Structural analyses revealed that both E-particles and F-particles are in the stage of closure, share high similarities in their surface structure, and demonstrate ordered epitopes, showing the potential to be ideal vaccine candidates. The findings were identical with the results of immunological experiment on cells and animals. A structural comparison between E30 and EV-A, EV-B and EV-C species showed that viral protein 1 (VP1) BC loop of them exhibits no conservation, indicating that it can be used to tell the differences of important structural traits of various enterovirus serotypes. Meanwhile, their VP1 GH loop and VP2 EF loop are relatively conservative, showing that they can be used to design broad-spectrum antibodies of EV-B species.
Researchers also analyzed the structures in complex of E30 in the resolution of 3.3 angstroms and 3.6 angstroms and its uncoating receptor human neonatal Fc receptor (FcRn) and that of E30 and its decay-accelerating factor (DAF/CD55), showing the structural basis of E30 in the recognition and combination of specific receptors. Further studies found that VP1 EF loop, VP1 GH loop and VP2 EF loop jointly build molecular foundation for enterovirus and its uncoating receptor recognition. By computing all pairwise distances in different viral target regions, researchers obtained K-vector and distance matrix between different viruses and came up with the computing method to predict the uncoating receptor of enterovirus (Figure 1).
To get better ideas for designing drugs and vaccines, researchers selected two highly neutralizing and potent E30-specific monoclonal antibodies, 6C5 and 4B10. Both the surface plasmon resonance (SPR) experiment and real-time PCR experiment showed that 6C5 and 4B10 can neutralize E30 infection efficiently by blocking viral binding to its two types of receptors. Interestingly, relevant experiments also revealed that they can coordinate and complement each other, indicating that they will produce additional effects when used together. High-resolution structures of E30-6C5-Fab and E30-4B10-Fab revealed that 6C5 and 4B-10 engage the capsid loci at the rim of the canyon and in-canyon respectively. The comformational epitope, which consists of two neutralizing antibodies, is relatively active in EV-B species. The above-mentioned structural information of atomic resolution provides important reference to the design of broad-spectrum vaccines and drugs mainly in conservative regions while avoiding non-conservative areas.
Prof. WANG Xiangxi, Academician RAO Zihe and Researcher ZHU Fengcai are corresponding authors of the two research papers. Their co-first authors include WANG Kang, a doctoral student jointly trained by Nankai University and the IBP, Dr. CUI Lunbiao and Dr. ZHANG Li, both of whom are from Jiangsu Provincial Center for Disease Control and Prevention. The research programs have received great support from the research team of Academician GAO Fu with the CAS Institute of Microbiology and Researcher ZHAI Weiwei with the CAS Institute of Zoology. In addition, the Strategic Priority Research Program of the CAS, the Innovation Group Members of National Natural Science Foundation of China, National Key Research and Development Program of China with the Ministry of Science and Technology, and Beijing Haidian Union Fund have given their assistance as well.
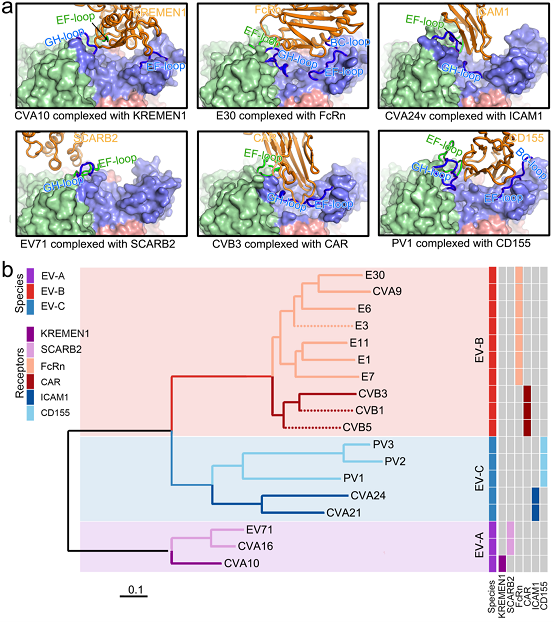
Figure 1: Structural foundation of the combination of virus and receptor, and the development of computing method to predict receptor on the basis of structure
(Image by Dr. WANG Xiangxi)
The web links for two research papers:
https://www.nature.com/articles/s41467-020-18251-9
https://www.nature.com/articles/s41467-020-18250-w
Contact: WANG Xiangxi
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: xiangxi@ibp.ac.cn
(Reported by Dr. WANG Xiangxi)

