Research Teams Make Progress in Studying the Packaging Mechanism of COVID-19
The COVID-19 disease, a novel coronavirus-caused pneumonia, has become a worldwide pandemic. It has exerted great influence upon economic growth and social stability because of the absence of effective measures concerning the prevention and treatment of the communicable disease.
After the invasion into host cells, the virus will make transcription of their genomes and translate into a number of structural and non-structural proteins. As one of structural proteins, nucleocapsid (N) protein can bind to viral genome RNA and pack it into 100 nm particles with the assistance of membrane (M) and envelope (E) proteins before eventually releasing them out of host bodies. The molecular mechanism by which N protein binds to and packs up viral genome RNA still remains unknown.
Together with the team of CHEN Wei from Zhejiang University, the team of LOU Jizhong with the Institute of Biophysics (IBP) under the Chinese Academy of Sciences published a research article titled "Liquid-liquid phase separation by SARS-CoV-2 nucleocapsid protein and RNA" in the journal Cell Research on September 9, 2020. They found the liquid-liquid phase separation (LLPS) between coronavirus N proteins and single-stranded RNAs (ssRNAs) and explored the mechanism behind its occurrence and regulation. According to their research, the LLPS can facilitate viral packaging.
After analyzing the sequence of coronavirus N protein, researchers found that there were many disordered regions with positive charge in N protein, which can bind to ssRNA. Its N-terminal RNA-binding domain (NTD) is the binding site of nucleic acids, while its C-terminal domain (CTD) can mediate dimerization. In other words, N protein has had typical features of the LLPS. N protein has been confirmed to undergo the LLPS with ssRNA. The LLPS is dependent on the length of ssRNA, while having no obvious ties with ssRNA-specific sequence.
Through further studies on truncated proteins, the researchers found that the CTD of N protein is of great importance to the LLPS with ssRNA. The absence of the binding sites of nucleic acids has had little influence in the process, indicating that N protein exists in other binding sites of nucleic acids. In addition, they also tested a number of metal ions and concluded that zinc ions can facilitate the LLPS of N protein with ssRNA. Zinc ions act as second messenger in cells and play an important role in the regulation of physiological function of cells.
The LLPS between N protein and ssRNA helps the public learn more about the packaging process of novel coronavirus. The regulatory function played by zinc ions can be applied to the prevention and clinical treatment of the COVID-19.
The research has been jointly done by the Institute of Biophysics of the Chinese Academy of Sciences and Zhejiang University. CHEN Hui, a postdoctoral student jointly supervised by the Institute of Biophysics of the Chinese Academy of Sciences and Shenzhen Baoan Maternal and Child Health Hospital, is the first author of the published article. Researcher LOU and Prof. CHEN Wei are the corresponding authors of the article. WANG Peihui, a professor with Shandong University, has contributed to the research as well.
The research has received assistance from the National Key Research and Development Program of China of the Ministry of Science and Technology and the National Natural Science Foundation of China, together with XU Tao, ZHANG Hong and SUN Fei, three researchers from the Institute of Biophysics of the Chinese Academy of Sciences.
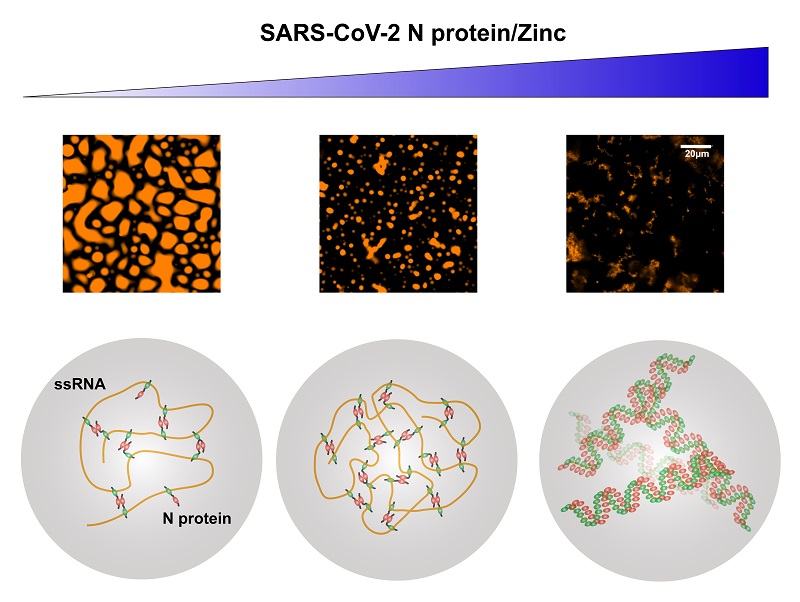
Figure: The liquid-liquid phase separation (LLPS) between coronavirus N proteins and ssRNAs
(Image by Dr. LOU Jizhong's group)
The web link for the research article: https://www.nature.com/articles/s41422-020-00408-2
Contact: LOU Jizhong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: jlou@ibp.ac.cn
(Reported by Dr. LOU Jizhong's group)

