WANG Jiangyun's Group Makes Progress in Studying the Preference of GPCR Signal Transduction
G Protein-Coupled Receptors (GPCRs) are an important target in pharmaceutical research. The GPCRs are the direct target of roughly 30 percent of clinical prescription drugs in over 800 human genomes. It is of great significance to figure out the conformational changes of the GPCRs as such changes always occur when GPCRs work.
Noticeable progress has been made in the research methods of structural biology, laying a solid foundation for studying the functions of the GPCRs in recent years. Common approaches available to study the structure of the GPCRs include crystallography, nuclear magnetic resonance (NMR), and cryo-electron microscopy (cryo-EM). The information collected via the technologies such as NMR and cryo-EM can only allow researchers to understand the structure and functions of the GPCRs in the static state. Therefore, it is difficult for them to apprehend the conformational dynamics of the GPCRs and the instantaneous regulation when they interact with downstream proteins.
Together with the group of Professor SUN Jinpeng from Shandong University School of Basic Medical Sciences (BMS), the group of Researcher WANG Jiangyun with the Institute of Biophysics (IBP) at the Chinese Academy of Sciences published a research article titled "DeSiphering receptor core-induced and ligand-dependent conformational changes in arrestin via genetic encoded trimethylsilyl 1H-NMR probe" in the journal Nature Communications on September 25, 2020.
Participants employed the highly efficient and sensible NMR technique to explore trimethylsily phenylalanine (TMSiPhe) and 950 MHz 1D 1H-NMR and explained the functional mechanism where litigants selectively accommodate to the conformational changes of arrestin in the core regions of the GPCRs transmembrane helices.
Researchers utilized the technique of genetic code expansion to label site-specific incorporation of 4-TMSiPhe into proteins, uncovered the selective accommodation mechanism of the TMSiPheRS to the TMSiPhe through crystallographic analysis, and employed the method of 950 MHz 1D 1H-NMR to depict the multiple conformational state of downstream arrestin protein under the regulation of the GPCR in different litigants.
Owing to unique up-field 1H-NMR chemical shift and the highly efficient incorporation of TMSiPhe, high-quality NMR spectra can be acquired by using only 5 μM of protein and 10 minutes of spectrum accumulation time. The simple and efficient method can be widely applicable to the studies of conformational changes of high molecular membrane protein complexes.
First-authors of the paper are LIU Qi, HE Qingtao, two doctoral students with the BMS at Shandong University, YANG Fan, a postdoctoral student with the BMS, LYU Xiaoxuan, a postdoctoral student with the IBP, and ZHU Zhongliang, a senior engineer with the University of Science and Technology of China. Professor SUN Jinpeng and Researcher WANG Jiangyun are its correspondence authors.
The research has received assistance from the National Key Research and Development Program of China of the Ministry of Science and Technology and the National Natural Science Foundation of China.
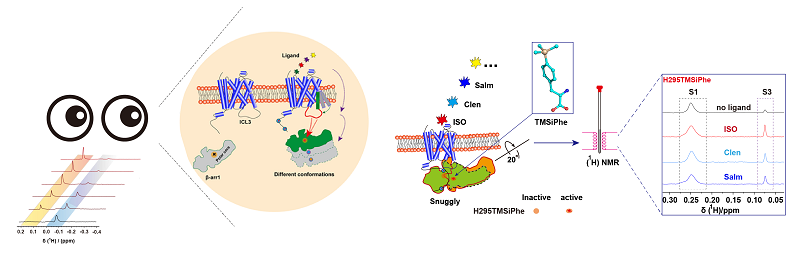
Figure: TMSiPhe and 950 MHz 1D 1H-NMR have been used to measure multiple conformational state of arrestin protein under the regulation of the GPCR in different litigants.
(Image by Dr. WANG Jiangyun's group)
The link for the research article: https://www.nature.com/articles/s41467-020-18433-5
Contact: WANG Jiangyun
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: jwang@ibp.ac.cn
(Reported by Dr. WANG Jiangyun's group)

