Researchers Reveal Molecular Mechanism of Herpesvirus to Inhibit mRNA's Nuclear Export of the Host
The virus has developed many mechanisms to confront and escape the antiviral response of the host, among which by intervening the host's mRNA nuclear export process to prevent host cells from establishing an appropriate antiviral environment is a key tactic.
In 2016, a study found that the ORF10 protein encoded by γ herpesvirus can inhibit parts of the host's mRNA nuclear export by interacting with Rae1-Nup98, a transport compound of the host's mRNA.
ORF10 is the first virulence protein found in herpesviruses to inhibit the host's mRNA nuclear export, which is also the first reported viral protein that selectively inhibits specific mRNA transport.
GAO Pu and DENG Hongyu research groups, from the Institute of Biophysics,Chinese Academy of Sciences (CAS) jointly released a research paper named "Molecular mechanism underlying selective inhibition of mRNA nuclear export by herpesvirus protein ORF10" on PNAS on October 8, 2020, analyzing the interactive details of ORF10 with the transport compound Rae1-Nup98, identifying ORF10's RNA integration capabilities and key functions and revealing the mechanism of ORF10's selective inhibition to mRNA's nuclear export.
The analysis found that ORF10 and Rae1-Nup98 compound have two interactive surfaces where two amino acids L60 and M413 of ORF10 contribute the important hydrophobic interaction. Researchers identified the importance of L60 and M413 in maintaining the interaction of ORF10 and Rae1-Nup98 and the nuclear export function of ORF10's mRNA.
By biochemical test, researchers confirmed that ORF10 can directly combine with RNA. The interaction of ORF10 and RNA is very crucial to inhibit mRNA's nuclear export function. They also analyzed the ternary complex structure of ORF10 protein and compound Rae1-Nup98, identifying that two important interactive surfaces of virus and host and the key amino acids and revealing ORF10's ability of directly combining RNA and the importance of inhibiting host function.
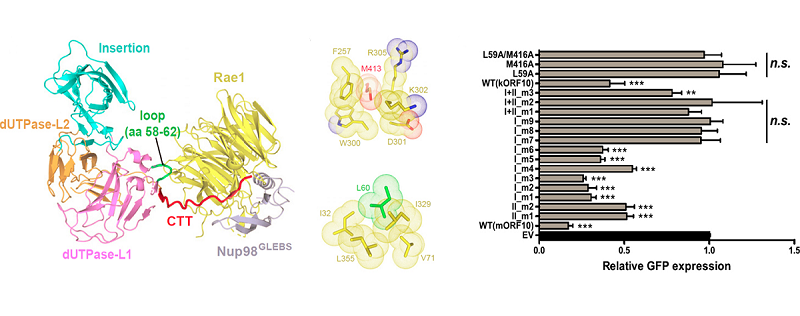
Impact of the structure, interactions and mutants of ORF10 and Rae1-Nup98 compound on GFP expression
(Image by Dr. GAO Pu's groups)
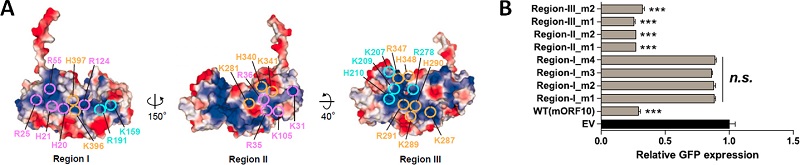
The impact of ORF10's surface charge distribution and mutants on GFP expression
(Image by Dr. GAO Pu's groups)
Researchers GAO Pu and DENG Hongyu are the joint correspondence authors of the paper. FENG Han, associate research fellow of GAO's research team and TIAN Huabin, research assistant of DENG's are the co-first authors. The research work also got the supports from the Ministry of Science and Technology, the National Natural Science Foundation and the CAS.
Article Link: https://www.pnas.org/content/early/2020/10/07/2007774117
Contact: GAO Pu, DENG Hongyu
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: gaopu@ibp.ac.cn, hydeng@ibp.ac.cn
(Reported by Dr. GAO Pu and DENG Hongyu's groups)

