Researchers Reveal Structural Basis for Two Metal-Ion Catalysis of DNA Cleavage by Cas12i2
Clustered regularly interspaced short palindromic repeats CRISPR-Cas (CRISPR-associated proteins) is an RNA-guided adaptive immunity system present in bacteria and archaea. Cas9 and Cas12a, two Cas proteins used most in gene-editing, both encompass a RuvC catalytic domain.
To understand how the RuvC domain cleaves DNA, it is critical to elucidate the structures of RuvC-containing Cas complexes in their catalytically competent states, with both metal-ions and ssDNA substrate bound in the RuvC catalytic pocket.
A research team led by Scott W. G. et al discovered a Class 2 type V-I CRISPR-Cas "Cas12i2" in December 2018. With molecular weight lighter than Cas12a and Cas9, Cas12i2 is expected to be an emerging tool for gene-editing. It is critical to learn more about its structure and catalytic mechanism.
In a study published in Nature Communications online on October 16, 2020, researchers from the Institute of Biophysics (IBP) of Chinese Academy of Sciences (CAS) and the University of Science and Technology of China (USTC) revealed the crystal structures of Cas12i2-crRNA complexes and Cas12i2-crRNA-DNA complex, the mechanism of DNA recognition and cleavage by Cas12i2, and activation of the RuvC catalytic pocket induced by a conformational change of the Helical-II domain. Together, their studies provided significant insights into the DNA cleavage mechanism by RuvC-containing Cas proteins.
Researchers reported high-resolution structures of Cas12i2-crRNA in three states, including the binding state, the seed region pairing state and the catalytic state. These data suggest that the crRNA:DNA duplex of 13-nt and longer has the ability to initiate cleavage, Helical-II domain conformational change activates the RuvC domain.
They captured the catalytic state of Cas12i2, with both metal-ions and the ssDNA substrate bound in the RuvC catalytic pocket, which has revealed the essential roles of metal-ions for DNA cleavage by the RuvC domain in Cas12 and Cas9. It is also the first time where an ssDNA and two metal-ions were observed in the RuvC catalytic pocket, showing their active state.
Besides, their structural and biochemical characterization of Cas12i2 provided insights into the molecular mechanisms of this CRISPR effector and suggests potential applications of Cas12i2. Researchers observed that pre-crRNA processing has effects on dsDNA cleavage activity.
Their biochemical studies showed that Cas12i2 cleaves ssDNA in a PAM-independent manner. Furthermore, the formation of the crRNA:target DNA hybrid activates the RuvC catalytic pocket, with base-pairing in the seed region not being essential for activation. Their findings had implications for developing more reliable genome-editing or nucleic acids detection tools.
Correspondence authors are WANG Yanli and SHENG Gang of the IBP and Prof GONG Weimin of the USTC. HUANG Xue is the first author. The work was supported by grants from the Chinese Ministry of Science and Technology, the Natural Science Foundation of China and the Chinese Academy of Sciences. Shanghai Synchrotron Radiation Facility and Spring-8 also provided key technical support.
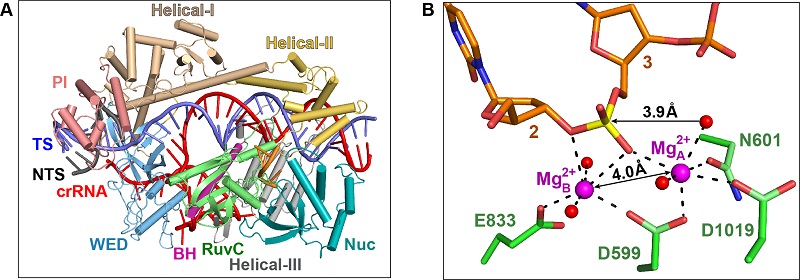
Structural and biochemical characterization of Cas12i2
A. Crystal structures of the 12-nt DNA-bound Cas12i2 ternary complex
B. The substrate and two metal cations in the catalytic pocket of Cas12i2
(Image by Dr. WANG Yanli's group)
Article Link: https://www.nature.com/articles/s41467-020-19072-6
Contact: WANG Yanli
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: ylwang@ibp.ac.cn
(Reported by Dr. WANG Yanli's group)

