Ferrous Iron and Polysulfide Coordinated Ferroptosis-Like Death in Bacteria for Anti-Infection Therapy
Their latest findings were published recently in Nano Today with the title of "Nano-decocted ferrous polysulfide coordinates ferroptosis-like death in bacteria for anti-infection therapy".
Ferroptosis is an iron-dependent oxidative cell death, different from apoptosis, necrosis and autophagy. It is featured with lipid peroxidation induced by iron or lipoxidase, together with an exhaustion of glutathione (GSH). In addition, it can lead to a contraction of mitochondria and an increase in membrane density. Many studies have shown that ferroptosis is closely related to neurological disorders, tumors and heart diseases. Therefore, further research will deepen our understanding of the developmental mechanism of those diseases and provide guidance to drug discovery. Current studies on ferroptosis mainly focus on eukaryotic systems such as the mammalian and plant cells, which are regulated precisely by signal pathways such as GPX 4, system XC- or p53. However, such ferroptosis has not been found in bacteria or other prokaryotic systems.
In 2007, Prof. GAO found that iron oxide nanozymes have enzyme-like activity similar to peroxidase (Nature Natotechnology 2007). He then applied these nanozymes (iron oxide and iron sulfide) to remove oral biofilm of bacteria (Biomaterials 2016, Nature Communications 2018) and eliminate intracellular Salmonella (Theranostics 2018).The researchers found that some nanozymes can produce considerable antibacterial effects without the presence of hydrogen peroxide. In other words, the utility of iron sulfide nanozymes alone can eliminate various bacteria in an efficient manner. However, little is known about the mechanism behind the phenomenon. According to their early investigations, iron sulfide nanozymes can produce hydrogen polysulfide which was assumed to be critical for bacteria killing, however, the use of polysulfide alone has a limited effect.
Apart from expounding the biological mechanism of how iron-based nanozymes alone eliminate bacteria, the researchers found for the first time that bacteria can be killed in a ferroptosis-like pattern. Iron sulfide nanozymes such as Fe3S4. can generate hydrogen sulfide and a large amount of iron to form a solution featured with ferrous iron and polysulfide when they undergo oxygen-sulfide exchange reaction in water.
To better investigate the anti-bacteria property of iron sulfide nanozymes, a nano-decoction was made from the aqueous suspension through high-pressure sterilization procedure and centrifugation similar to the procedure of preparing traditional Chinese medicines. Such nano-decoction can kill up to 90 percent of Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) within five minutes, while it took antibiotics such as vancomycin about 24 hours to achieve an equivalent antibacterial effect.
Further studies showed that the nano-decoction can destroy the integrity of bacteria, lead to a leakage of cytoplasm content, trigger lipid peroxidation, and suppress the activity of complex I and II in the respiratory chain of bacteria. Transcriptome analysis showed that multiple metabolic pathways have been restrained. In addition, the oxidation of GSH into GSSG (oxidized glutathione) led to exhaustion of glutathione in the elimination of bacteria.
These features indicated that such a death was similar to ferroptosis. Furthermore, ferroptosis inhibitors such as Ferrostatin-1 were found with the ability to suppress bacterial death induced by nano-decoction. Iron chelators such as EDTA and ATP can exercise similar inhibitive effects in the process. Biochemical experiment showed that nano-decoction can directly oxidize GSH into GSSG, in which the polysulfide plays a major role to react with thiol in GSH.
Based on above-mentioned findings, researchers thus belived that ferrous iron and polysulfide in nano-decoction coordinately induced bacterial death with hallmarks similar to ferroptosis. It is noted that such a ferroptosis-like death in bacteria depends on ferrous iron rather than ferric iron. It is only applicable to bacteria, has no effect on fungi. Of noted, the ferrous iron only achieves an optimal effect in aqueous solution. The anti-bacteria effect has been curtailed because of the existence of chelators and GSH in relevant culture medium.
Interestingly, the nano-decoction showed the ability to suppress intracellular S. aureus and MRSA (methicillin-resistant S. aureus) in macrophages without triggering cytotoxicity to host cells. Ferrous iron and polysulfide from nano-decoction can restrain bacteria but fail to induce cellular ferroptosis when they enter into macrophages. The researchers attributed different consequences to the fact that the antioxidant system of bacteria is simple and easier to be killed, while eukaryotic system of cells possesses powerful antioxidant system and higher GSH level which can thus resist the simulation of nano-decoction.
Animal experiments demonstrated that intravenous injection of nano-decoction can dramatically reduce S. aureus or intracellular in blood and prolong the survival period of septic mice. More importantly, nano-decoction showed an equivalent therapeutic effect to vancomycin for infectious pneumonia treatment induced by S. aureus.
Researchers believed that bacteria can be killed in a ferroptosis- like manner. Combining with previous findings related to the mechanism in which iron oxide nanozymes induce lipid peroxidation in the virus of lipid envelope and viral disinfection (Theranostics 2019), they speculated that ferroptosis can happen to both bacteria and viruses, though there is no relevant signal pathway for regulation. Nevertheless, such a channel can still be used to develop new anti-bacterial and anti-virus drugs.
Moreover, the ferroptosis-like death in bacteria is probably a common mechanism for various inorganic antibacterial inorganic nanomaterials and thus can act as a guidance for the development of antibacterial materials.
The research was completed by the Institute of Biophysics of Chinese Academy of Sciences and Yangzhou University. Prof. GAO Lizeng is corresponding author of the published paper. It was supported by the National Key R&D Program of China and the National Natural Science Foundation of China.
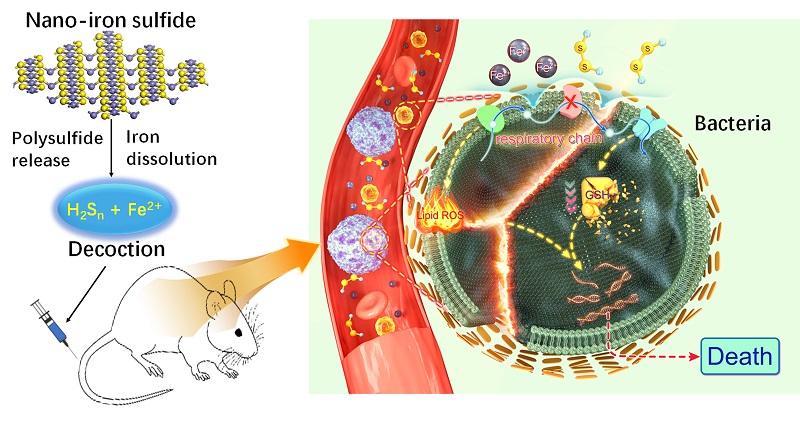
Ferrous iron and polysulfide coordinate bacterial death with hallmarks similar to ferroptosis for anti-bacterial treatment
(Image by Dr. GAO Lizeng's group)
Article link:https://www.sciencedirect.com/science/article/pii/S174801322030150X
Contact: GAO Lizeng
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: gaolizeng@ibp.ac.cn
(Reported by Dr. GAO Lizeng's group)

