Study Reveals Drug Entry Channel in Ferritin Nanocage
Ferritin, a naturally expressed iron binding protein in human cells, plays crucial roles in the maintenance of iron balance and the resistance of cellular oxidation. With a 12 nm outer diameter shell and 8 nm diameter hollow cavity, composed of 24 self-assembly subunits, ferritin has emerged as a promising drug delivery vehicle due to its unique architecture, intrinsic tumor targeting property, and excellent biocompatibility. However, the mechanism of drug loading in ferritin nanocage is obscure.
A research group led by Prof. YAN Xiyun from the Institute of Biophysics of Chinese Academy of Sciences (CAS) recently published their latest finding revealing the mechanism of drug loading for ferritin drug carrier (FDC) in Nano Today. The paper reported the structure basis of drug entry channels on the surface of FDC and provided theoretical foundation to the clinical translation of ferritin drugs.
In their previous work, the researchers, who are also affiliated to the CAS Engineering Laboratory for Nanozyme, found that recombinant human ferritin heavy chain is a novel ligand of transferrin receptors (TfR1), which is a well-known tumor biomarker, and developed a mild method to effectively loading drugs into ferritin nanocage. Moreover, they also came up with the concept of FDC.
Similar to antibody-drug conjugates (ADC), FDC can specifically deliver drugs to tumor tissues via binding to receptors. In addition, FDC is superior to ADC in several aspects such as drug-loading capacity, thermal stability, and easier access to production. Nevertheless, the translation studies of ferritin drug carrier are impeded by the low efficiency and low yield of the drug loading process.
Through crystal structure and protein mutation analysis, Prof. YAN Xiyun and her team identified a unique thermal-sensitive drug entry channel in HFn protein nanocage that could be utilized in efficiently loading small molecule chemotherapeutic drugs. The residues at the 43, 92, 91, 90, and 89 from one subunit, and residues at the 81, 79 from the neighboring subunit constitute the drug entry channel, of which the 'open' state is controlled by temperature.
Through drug loading by this process, the FDC possessed better stability, increased biosafety, and enhanced antitumor activity than those of HFn-Dox prepared by denaturation-based methods. Intriguingly, the drug enter channel appears quite unique to HFn, and could be used for other small molecule drugs. It is conceivable that additional chemotherapeutic drugs, or combination of them might be loaded into the HFn cages for effective anti-cancer treatment , which will greatly facilitate the translational study of ferritin drug carriers in cancer-targeting therapies.
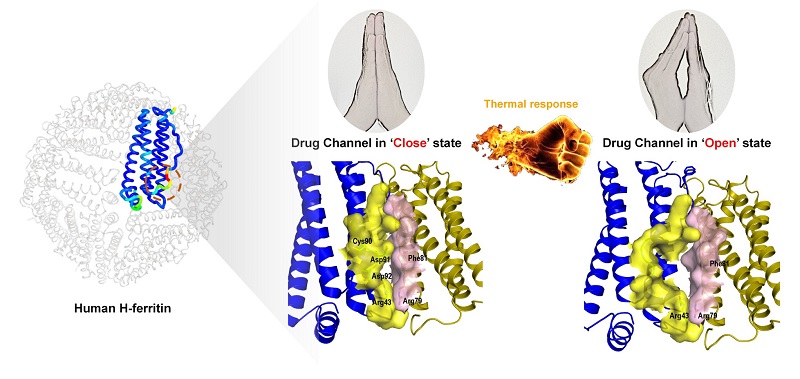
The opening state of ferritin drug loading channel is sensitive to temperature changes
(Image by Dr. Fan Kelong's group)
Prof. YAN Xiyun and Prof. FAN Kelong are corresponding authors of the publication, while JIANG Bing, a doctoral fellow with the School of Basic Medical Sciences at Zhengzhou University, and CHEN Xuehui, a doctoral fellow with the Institute of Biophysics of Chinese Academy of Sciences, are its first-authors.
The research was financed by the National Natural Science Foundation of China, the Strategic Priority Research Program of the CAS and other funds. Shanghai-based Synchrotron Radiation Facility also extended its hand to researchers in the process.
Article Link: https://www.sciencedirect.com/science/article/pii/S1748013220301171
Contact: FAN Kelong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: fankelong@ibp.ac.cn
(Reported by Dr. FAN Kelong's group)

