Dr. ZHU Bing's group discovered TNF-α mediated inflammatory transcriptional memory and revealed its molecular mechanism
On November 6, 2020, eLife published a research article entitled "Sustained TNF-α stimulation leads to transcriptional memory that greatly enhances signal sensitivity and robustness" online. This work was accomplished by Dr. ZHU Bing's group at the National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences. In this work, the authors discovered that sustained TNF-α stimulation can induce transcriptional memory, which offers more rapid, more strong and more sensitive subsequent TNF-α response for certain memory genes. This TNF-α mediated transcriptional memory is dependent on signal induced transcription factor activation and active DNA demethylation.
Signals can activate specific genes to control various biological processes. Most genes respond to previously experienced signals similarly to the first response. However, certain genes respond to previously experienced signals more robustly. This phenomenon is termed as transcriptional memory. The proinflammatory cytokine TNF-α (tumor necrosis factor-α) activates NF-κB pathway and plays a vital role in the pathogenesis of chronic inflammatory diseases. Targeted inhibition of TNF-α has achieved remarkable therapeutic success in patients with chronic inflammation, such as rheumatoid arthritis and ulcerative colitis.
Does the key cytokine TNF-α in the chronic inflammation mediate transcriptional memory? If so, what is the detailed mechanism? Which epigenetic enzymes and chromatin modifications play the determinant roles in the establishment of mammalian transcriptional memory? Moreover, what differentiates genes with or without transcriptional memory effects? Addressing these questions will promote our understanding regarding transcriptional memory and chronic inflammation.
Using HEK293F cells containing an integrated DNA methylation silenced reporter gene as a model system, Dr. ZHU Bing's group found that sustained TNF-α stimulation can induce the transcriptional memory of the reporter gene, which stimulates more strong and more rapid induction in the subsequent TNF-α stimulation. Then the authors identified a series of endogenous genes exhibiting TNF-α mediated transcriptional memory. Among them, the best endogenous memory gene is CALCB, which encodes the migraine therapeutic target CGRP.
The DNA demethylation of the transcription factor NF-κB occupied genomic regions is accompanied with the establishment of the transcriptional memory. In the TET triple knockout (TKO) cells, which are depleted of active DNA demethylation, all the memory genes lost the TNF-α mediated transcriptional memory effect. These data suggest that active DNA demethylation is indispensable for consolidating transcriptional memory.
However, not all the NF-κB target genes exhibit transcriptional memory in response to TNF-α induction. High CpG density and initial methylation levels around the NF-κB binding sites correlate with the functional potential to serve as transcriptional memory modules.
Interestingly, for a few memory genes (e.g., CALCB), the memory consolidated cells can achieve a better induction with a 125-fold lower TNF-α concentration (0.4 ng/mL) than naive cells treated with 50 ng/mL TNF-α. This data suggest that the establishment of transcriptional memory could significantly elevates the cells' sensitivity to the inflammatory signal. This phenomenon may benefit the understanding for the transition from acute to chronic inflammation.
In conclusion, this study discovered the inflammatory cytokine TNF-α induced transcriptional memory, which is dependent on NF-κB pathway and active DNA demethylation. Such transcriptional memory offers more rapid, more strong and more sensitive subsequent TNF-α response. This is likely a general principle that can be applied to other signaling systems. This study offers a pipeline to systematically identify genes with transcriptional memory.
Prof. ZHU Bing is the corresponding author. Dr. ZHAO Zuodong and Dr. ZHANG Zhuqiang are the co-first authors. LI Jingjing, Dr. DONG Qiang, Dr. XIONG Jun, Dr. LI Yingfeng, LAN Mengying from ZHU Bing laboratory and Prof. LI Gang from the university of Macau made important contributions to this work. This work is supported by the National Natural Science Foundation of China, the Ministry of Science and Technology of China, and the Chinese Academy of Sciences.
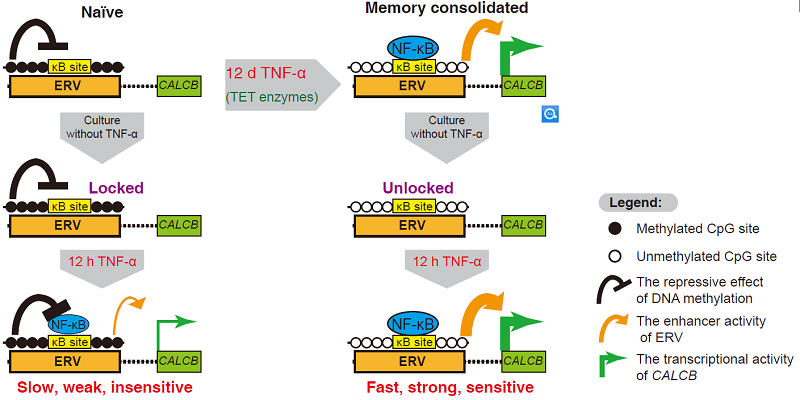
Fig1:Mechanisms of TNF-α mediated transcriptional memory (e.g., CALCB)
(Image by Dr. ZHU Bing's group)
The web link of this paper: https://elifesciences.org/articles/61965
Contact: ZHU Bing
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: zhubing@ibp.ac.cn
(Reported by Dr. ZHU Bing's group)

