Research Reveals Inositol Polyphosphate Multikinase Inhibits Liquid-Liquid Phase Separation of TFEB to Negatively Regulate Autophagy Activity
A research group led by Prof. ZHANG Hong from the Institute of Biophysics of Chinese Academy of Sciences (CAS) revealed that inositol polyphosphate multi-kinase negatively regulates autophagy activity. This study was published in Developmental Cell online on November 11, 2020.
Protein liquid-liquid phase separation (LLPS) has been shown to mediate the formation of a variety of membrane-less compartments. LLPS-mediated assembly of transcriptional condensates have recently been implicated in gene expression. Little is known about how TFs are prevented from undergoing homotypic and heterotypic interactions with other components of the transcriptional apparatus in the absence of transcriptional stimuli.
Autophagy involves sequestration of a portion of cytoplasmic contents such as protein aggregates and damaged organelles in a double-membrane autophagosome and its subsequent delivery to lysosomes for degradation. To understand how autophagy activity is controlled during animal development, researchers performed forward genetic screens and identified the ipmk-1(bp1075) mutation, which promotes autophagy activity during C. elegans development. ipmk-1 encodes the ortholog of mammalian inositol polyphosphate multikinase (IPMK).
Researchers revealed that IPMK depletion promotes autophagy and lysosomal function and biogenesis in a TFEB (Transcription Factor EB)-dependent manner. They found that nuclear TFEB forms distinct puncta with liquid-like properties. Nuclear TFEB puncta colocalize with the Mediator complex and also with mRNAs of target lysosomal genes. TFEB undergoes LLPS in vitro. IPMK directly interacts with and inhibits LLPS of TFEB, and also dissolves TFEB condensates. Depletion of IPMK increases the number of nuclear TFEB puncta and the colocalization of TFEB with Mediator and mRNAs of target genes.
In summary, this study revealed a novel mechanism for autophagy regulation in which nuclear IPMK acts as a chaperone to modulate LLPS of TFEB.
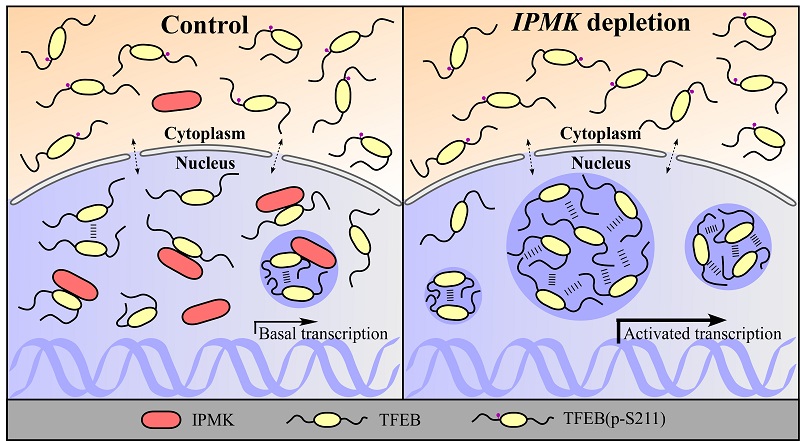
Figure. A model showing that IPMK regulates autophagy activity by modulating LLPS of TFEB
(Image by Dr. ZHANG Hong's group)
This research was financed by the National Natural Science Foundation of China, the Chinese Ministry of Science and Technology; the Beijing Municipal Science and Technology Committee; the Strategic Priority Research Program of the Chinese Academy of Sciences (CAS) and the Key Research Program of Frontier Sciences, CAS.
Article link: https://www.sciencedirect.com/science/article/pii/S1534580720308005?via%3Dihub=
Contact: ZHANG Hong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: hognzhang@ibp.ac.cn
(Reported by Dr. ZHANG Hong's group)

