Research reveals Molecular Mechanism of Membrane Remodeling by SNX1
Vesicle trafficking occurs between subcellular compartments in eukaryotic animal cells, which is responsible for cellular homeostasis. Coat proteins play a central role in the intracellular transport by coupling two major functions, bending membrane to generate transport carriers and binding to cargoes for their sorting into these carriers. Well-characterized coat protein complexes include COPI, COPII and Clathrin, while SNXs (Sorting Nexins) have been found to act as coat proteins in endosomal pathways that include recycling from endosomes to the plasma membrane and retrieval from endosomes to the Golgi complex, such as endosomal recycling of cargoes, including the cation-independent mannose-6-phosphate receptor and semaphorin 4C.
Researchers of SUN Fei's group from Institute of Biophysics of the Chinese Academy of Sciences, collaborating with Victor Hsu's group from Division of Rheumatology, Inflammation and Immunity of Brigham and Women's Hospital, and Department of Medicine of Harvard Medical School, have revealed how SNX1 is organized on membrane to explain its ability and mechanism to deform membrane by cryo-electron microscopy (cryo-EM) helical reconstruction technique.
Two helical assemble structures, with different diameters but similar assembling mechanism, were resolved. These structures show that there is one SNX1 dimer forming an asymmetric unit of the helical packing, oriented with its concave surface facing the membrane. Along the same helical row, two adjacent SNX1 dimers interact with each other through short lateral contacts formed by the BAR domain. The crosslinking of these parallel rows is mediated through the PX domain from one dimer in one helical row interacting with the BAR domain from another dimer residing in an adjacent helical row.
This work also has another discovery: the linker region between the BAR and PX domains of SNX1 plays an important role in membrane binding and tubulation, which is predicted to involve an amphipathic helix. Also the PI3P binding site plays a significant role in mediating electrostatic interactions with the negatively charged membrane surface.
They compared the SNX1 structure with a previously elucidated structure of an endosomal coat complex formed by retromer coupled to Vps5 (yeast homolog of SNX1), and reveals Vps5 exists in a more compact state when assembled into the retromer-SNX coat complex.
The work advances a molecular understanding of how a SNX deforms membrane. The comparison also suggests insight into intermediary stages of assembly that results in the formation of the retromer-SNX coat complex on membrane.
The work entitled "Structural insights into membrane remodeling by SNX1" has been published in Proceedings of the National Academy of Sciences.
The work was supported by the National Natural Science Foundation of China and the National Institutes of Health USA. All EM data were collected at Center for Biological Imaging, Institute of Biophysics, Chinese Academy of Sciences.
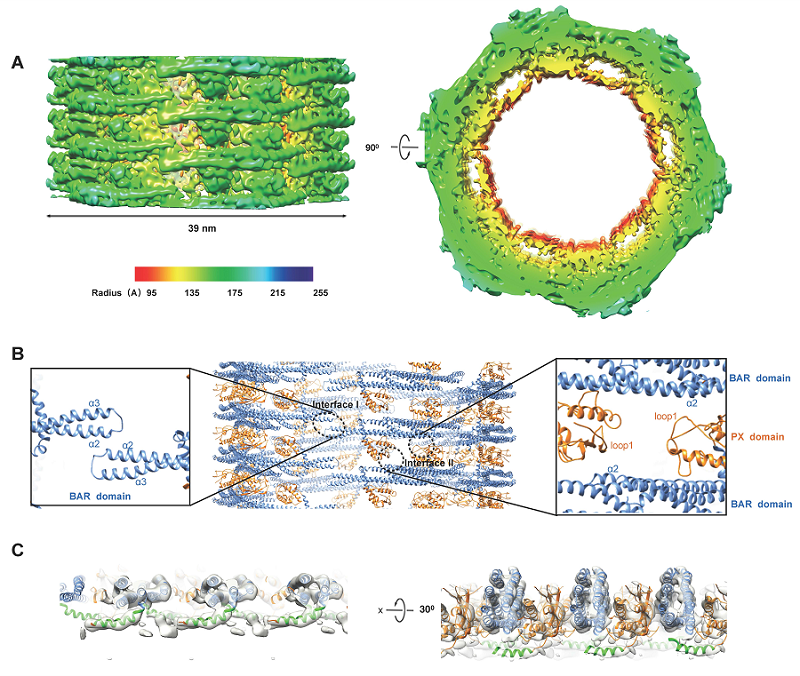
Cryo-EM structure of SNX1 coated tubules. A, Cryo-EM map of a class I tubule. B, Structural model of the SNX1 helical assembly. C, Ribbon model of the SNX1 dimer interacting with membrane, and the amphipathic helix is highlighted in green.
(Image by Dr. SUN Fei's group)
Paper link: https://www.pnas.org/content/118/10/e2022614118
Contact: SUN Fei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: feisun@ibp.ac.cn
(Reported by Dr. SUN Fei's group)

