IBP Researchers Develop New Bright pH-sensitive Fluorescent Proteins to monitor exocytosis docking and fusion steps
Vesicle secretion, especially the regulated exocytosis, regulates important physiological functions by controlling the release of peptides or neurotransmitters to the external of the cell in excitable cells such as neurons and endocrine cells. Since the 1960s, the Nobel Prize in Physiology or Medicine has been awarded 4 times to the field of vesicle secretion. However, although researchers have conducted extensive studies on vesicle secretion, there are still many unsolved questions associated with the molecular mechanisms. One of the main reasons is that the process of vesicle secretion is very complicated, which involves multiple steps associated with multiple protein molecules, and coupling events in time and space.
Vesicle exocytosis is a rapid and dynamic process. The current detection techniques for vesicle exocytosis mainly include electrophysiological and optical imaging methods. The patch clamp-based electrophysiological detection technology can detect the exocytosis of a single cell with high time resolution of the millisecond scale. However, this technology is mainly used for the detection of single cells in vitro or in brain slices, and it is not easy to achieve simultaneous detection of multiple cells and in vivo detection. In addition, the patch clamp technique detects the apparent event of exocytosis of multiple vesicles in a single cell, but cannot observe and detect the docking and fusion process of a single vesicle. On the other hand, the technology based on optical imaging can track and record vesicle exocytosis in multiple cells at the same time. Importantly, this technology can also detect the exocytosis of a single vesicle, which is very important for revealing the regulatory mechanism of vesicles and plasma membrane docking and fusion at the molecular level. In these processes, the fluorescent protein as fusion tag provides a powerful tool for microscopic imaging.
The mature vesicle cavity has an acidic pH environment (pH is about 5.6). During exocytosis, the vesicle membrane and the plasma membrane fuse, and the intraluminal pH instantly switches to an alkaline extracellular environment (pH approximately 7.4). Based on this characteristic, Miesenbock G. et al. developed a pH-sensitive GFP mutant and named pHluorin. Since pHluorin is almost completely quenched at pH 5.5, and the fluorescence intensity increases by 50 times at pH 7.5, it has been widely used to detect synaptic vesicles, secretory vesicles, and exocytosis of recirculating endosomes. However, the fluorescent signal of pHluorin in vesicles near the plasma membrane is very dim before membrane fusion, which limits its application in tracking the docking steps of vesicle secretion. On the other hand, compared with pHluorin, the existing red pH-sensitive fluorescent protein probes have worse pH sensitivity. Among these red pH-sensitive fluorescent proteins, pHuji has relatively good properties, but the protein has low fluorescence intensity and rapid fluorescence decay characteristics. The fluorescence intensity of pHuji drops to 35% of the initial fluorescence under continuous illumination of <2 s, which is not suitable for the detection of vesicle exocytosis and kinetic tracking.
To circumvent these problems, Professor XU Pingyong at the Institute of Biophysics (IBP) of the Chinese Academy of Sciences recently developed two new bright pH-sensitive fluorescent proteins, pHmScarlet0 and pHmScarlet.
pHmScarlet has higher brightness, stability and better pH sensitivity than pHuji. pHmScarle has the highest pH sensitivity among the existing red fluorescent proteins, which can detect more secretion events. Most importantly, it can visualize the vesicle docking and fusion steps in secretion events simultaneously. When used for detection of vesicle secretion, pHmScarlet has a bright exocytotic signal comparable to that of pHluorin. In addition, the pKa value of pHmScarlet makes it possible to detect the fluorescence intensity in intact vesicles under acidic conditions, enabling tracking and recording of vesicle docking steps, which cannot be performed using pHluorin. Meanwhile, the probe can be combined with pHluorin to perform dual-color imaging of multiple vesicle membrane proteins (Figure 1a-c). On the other hand, although the emission wavelength of pHmScarlet is significantly red-shifted compared with pHluorin, its spatial resolution is high enough for the super resolution imaging technique Hessian Structured Illumination Microscopy (Hessian-SIM) to resolve the ring structure of the vesicle fusion pore. It can be predicted that pHmScarlet will have a wide application in the field of vesicle secretion and transportation. Compared to pHmScarlet, pHmScarlet0 exhibits slightly lower pH sensitivity but two times more photostability and potential application of long-term imaging.
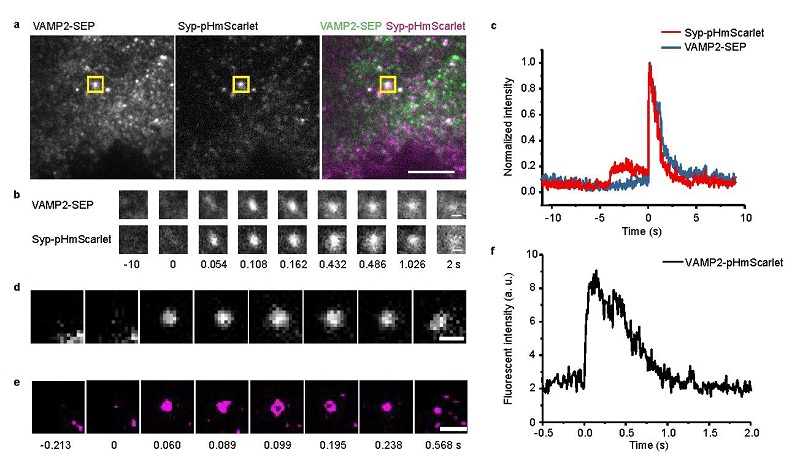
Figure 1 (a-c) Dual-color imaging of different vesicle membrane proteins labeled with pHmScarlet and pHluorin. (d-f) Super solution imaging of vesicle exocytotic event
(Image by Dr. XU Pingyong's group)
This work was done in cooperation with the Institute of Biophysics, Chinese Academy of Sciences, Peking University, and the Institute of Genetics and Development of the Chinese Academy of Sciences. Professor XU Pingyong and Associate Professor YUAN Lin at the Institute of Biophysics are the co-corresponding authors of this article.
The research work entitled "pHmScarlet is a pH-sensitive red fluorescent protein to monitor exocytosis docking and fusion steps" was published on line in the journal Nature Communications on March 3, 2021.
This project was supported by the National Key R&D Program of China, the National Natural Science Foundation of China and the Strategic Priority Research Program of Chinese Academy of Sciences.
Article Link: https://www.nature.com/articles/s41467-021-21666-7
Contact: XU Pingyong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: pyxu@ibp.ac.cn
(Reported by Dr. XU Pingyong's group)

