Research reveals molecular mechanism of a novel heme-copper terminal oxidase utilizing two electron donors
The heme-copper terminal oxidases (HCOs) superfamily, one of the most important metalloproteinases, is responsible for the efficient electron transfer from cytochrome c or quinol to molecular oxygen. The members of this family are multi-subunit complexes, such as 3 subunits in some bacteria and 14 protein subunits in mammalian mitochondria. In the complex, there is a conserved central catalytic subunit I, containing two heme groups and a copper atom (CuB), and the active site is formed by one high-spin heme and CuB. Subunit II usually contains binuclear CuA center. Cytochrome c oxidase (CcO) family belongs to the HCOs superfamily, but could only use cytochrome c as electron donor according to previous studies. Recently, a novel cytochrome c oxidase from the hyperthermophilic bacterium Aquifex aeolicus (AaCcO) was discovered to be able to use both cytochrome c and naphthoquinol (NQ) as electron donors, but its molecular mechanism as well as the evolutionary significance are still unknown.
Currently, researchers of SUN Fei's group from Institute of Biophysics of the Chinese Academy of Sciences, and Hartmut Michel's group from Max Planck Institute of Biophysics, solved the 3.4 ? resolution electron cryo-microscopic structure of AaCcO. This work not only revealed the molecular mechanism how this unusual oxidase could use both cytochrome c and quinol as electron donors, but also made structural insights into its thermal stability and the evolutionary adaptation of this oxidase to keep the balance between its enzymatic activity and structural stability for the hyperthermophilic growth condition.
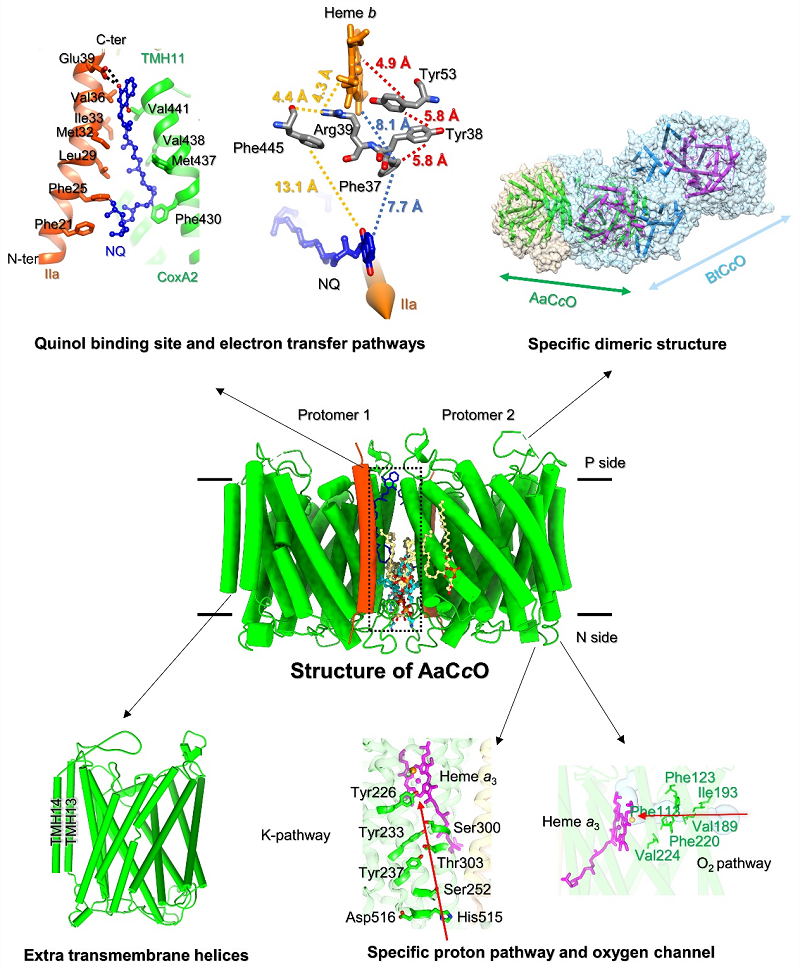
Structure of cytochrome c oxidase from Aquifex aeolicus (AaCcO)
(Reported by Dr. SUN Fei's group)
AaCcO forms a novel dimeric structure mediated by subunit I (CoxA2), and this dimeric form is different from that of all other reported CcO dimers. Fruitful protein-lipid interactions are observed in the dimeric interface. A novel substrate binding site of the naphthoquinol (NQ) is found at the dimeric interface, which could allow NQ be a direct electron donor bypassing cytochrome c. As a result, it seems that AaCcO dimer should be the necessary condition for direct electron transfer from NQ. Moreover, the adapted structure of AaCcO is found to have only one available proton pathway (K pathway) and a V-shaped unobstructed oxygen channel with more hydrophobic residues blocking one entry, which appeared to be evolutionary advantageous to keep the balance between its enzymatic activity and structural stability in the hyperthermophilic environment. These results provide structural basis for molecular mechanism and the evolutionary significance of cytochrome c oxidases in the extreme thermal environment.
The work entitled " The unusual homodimer of a heme-copper terminal oxidase allows itself to utilize two electron donors" has been published on line in Angewandte Chemie International Edition on March 4th, 2021.
The work was supported by Strategic Priority Research Program of Chinese Academy of Sciences, the National Key Research and Development Program of China and National Natural Science Foundation of China, etc. All EM data were collected at Center for Biological Imaging, Institute of Biophysics (IBP), Chinese Academy of Sciences (CAS). LC-MS analysis was completed at Lab of Proteomics, Core Facilities for protein Science, IBP, CAS.
Article Link: https://doi.org/10.1002/anie.202016785
Contact: SUN Fei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: feisun@ibp.ac.cn
(Reported by Dr. SUN Fei's group)

