JU Zhenyu/CHEN Chang team work to reveal a new mechanism of protein S-nitrosylation in regulating Hematopoietic stem cell regeneration
The group of JU Zhenyu at the Institute of Aging and Regenerative Medicine of Jinan University and the group of CHEN Chang at the Institute of Biophysics of the Chinese Academy of Sciences, revealed the effect of nitric oxide (NO) and protein S-nitrosylation (SNO) on regulating the proteostasis and viability of Hematopoietic stem cells during regeneration. On March 30, 2021 Beijing time, Cell reports published this research online.
Hematopoietic stem cell (HSC) is a stem cell in the blood system, which has the ability of long-term self-renewal and differentiation into a variety of mature blood cells. Chemotherapy, radiotherapy and other common methods of cancer treatment kill cancer cells and damage Hematopoietic stem cells, affecting blood regeneration and the patient's immune system. Under various stress conditions, especially in the case of bone marrow transplantation or chemotherapy-induced bone marrow ablation, the regenerative ability of Hematopoietic stem cells is very important for the supplement of Hematopoietic system. In order to maintain the long-term Hematopoietic reconstitution ability of Hematopoietic stem cells, most of the Hematopoietic stem cells are at rest and live in the environment of hypoxia. However, under the pressure of proliferation and self-renewal, the regulatory mechanism of oxidative stress and nitrification stress on HSC regeneration is still unclear.
Under the guidance of professor JU Zhenyu and CHEN Chang, the two groups of researchers jointly revealed the regulation mechanism of protein S-nitrosylation modification on the homeostasis and survival of HSC in the self renewal phase, and proved the mechanism of S-nitroglutathione reductase (GSNOR), the key enzyme of denitrosylation, mediated protein S-nitrosylation and Chop mediated unfolded protein reaction in regulating HSC activity during Hematopoietic stem cell regeneration. This finding highlights the importance of proper regulation of HSC protein homeostasis under proliferative stress, and the interference of protein S-nitrosylation modification may contribute to the functional expansion of HSC.
The researchers firstly found that the levels of NO and SNO in HSC under proliferation pressure were significantly increased, accompanied by the accumulation of a large number of protein aggregation. The results suggest that NO and SNO have a certain regulatory role in HSC under proliferation pressure. Using GSNOR knockout mice, we further found that GSNOR knockout had no significant effect on the phenotype of Hematopoietic system in steady state. However, under the pressure of proliferation, the ability of Hematopoietic reconstitution and self-renewal of HSC in GSNOR knockout mice decreased. Next, the researchers found that the levels of NO and overall SNO in GSNOR knockout HSCs were significantly increased by S-nitrosylation modified proteomics, mass spectrometry and flow cytometry, while the accumulation of cell aggregates and apoptosis were significantly increased. In order to prove the hypothesis that the over activated NO signaling pathway induces HSC remodeling defects in GSNOR knockout mice, the researchers treated the GSNOR knockout mice with L-NAME, an inhibitor of NOS, and found that the Hematopoietic reconstitution ability and self-renewal ability of GSNOR knockout mice were significantly enhanced. At the same time, treatment with TCA, a molecular chaperone for protein folding, and simultaneous knockout of chop gene in GSNOR knockout mice could significantly improve the Hematopoietic reconstitution ability of GSNOR knockout mice.
As a post translation modification, protein S-nitrosylation modification is an important mediator of signal transduction pathway. Abnormal S-nitrosylation usually affects protein misfolding and causes stress of endoplasm reticulum, which leads to cell function damage. The results showed that the protein S-nitrosylation and protein aggregation level increased under regeneration stress, which strengthened the relationship between the increase of protein S-nitrosylation and protein folding damage. In this study, the S-nitrosylation modification levels of HSP60, HSP70 and HSP90 (an important partner for protein folding) were significantly increased in knockout GSNOR cells. Next, the S-nitrosylation of HSP90 and its role in protein aggregation were determined. The new S-nitrosylation residues of HSP90 in the M domain of GSNOR knockout cells (cys521/590/591) were reported in this study. It was proved that HSP90 could affect the protein folding function and cause protein aggregation. The protein aggregation caused by GSNOR knockout can be improved by reversing the modification of S-nitrosylation by the nitrosylation residue of Hsp90. The results showed that the S-nitrosylation of Hsp90 damaged the protein folding activity, which resulted in the accumulation of protein aggregation in GSNOR knockout cells during stress. In view of the lack of GSNOR, protein aggregation can be induced by increasing the S-nitrosylation of many proteins, it is also very important to determine other important targets of GSNOR regulation of unfolded protein reaction.
This study revealed the important relationship between GSNOR mediated S-nitrosylation and chop mediated unfolded protein reaction in the regulation of HSC regeneration. This study emphasizes the importance of HSC protein homeostasis regulation in maintaining HSC stress activity under physiological and proliferative stress. At the same time, under the pressure of proliferation, the regulation of protein S-nitrosylation may contribute to the functional expansion of HSC without affecting its survival. This study provides a novel theoretical basis for the regulation of Hematopoietic stem cell regeneration.
Professor JU Zhenyu at Institute of aging and regenerative medicine of Jinan University, Professor CHEN Chang at Institute of biophysics of Chinese Academy of Sciences, Professor CHEN Zhiyang and Associate Professor LIU Bo of Institute of aging and regenerative medicine of Jinan University are the co corresponding authors of this paper. Drs YI Weiwei, ZHANG Yuying and associate professor LIU Bo are the co-first authors. This work was supported by the National Key R&D Program, the National Natural Science Foundation of China and the Strategic Priority Research Program of the Chinese Academy of Sciences.

GSNOR regulates the balance of protein S-nitrosylation (SNO) and NO in Hematopoietic stem cells under proliferative stress. In the reflection, when the bird is on the pile, the pile keeps balance. After the bird flies away, the pile becomes unbalanced. The bird represents GSNOR, and the balance of stakes is NO and SNO.
(Image by Dr. CHEN Chang's group)
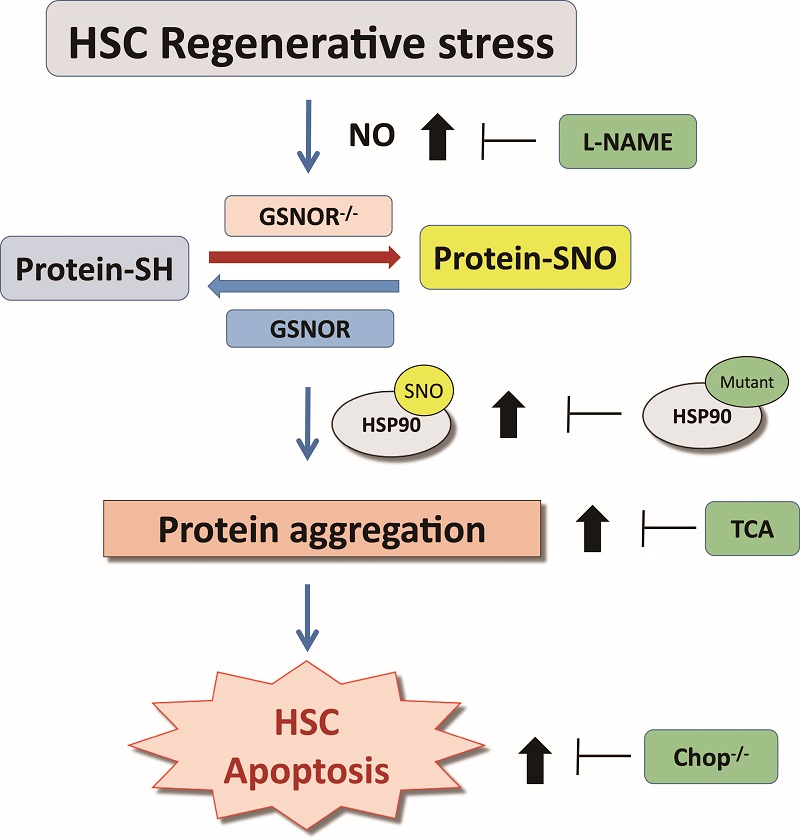
Graphical Abstract
(Image by Dr. CHEN Chang's group)
The article is available at https://doi.org/10.1016/j.celrep.2021.108922
Contact: CHEN Chang
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: changchen@ibp.ac.cn
(Reported by Dr. CHEN Chang's group)

