Cooperation of ER tubule-forming proteins, septin cytoskeletons and FIT2 in lipid droplet biogenesis
Lipid droplet (LD) is the major place for lipid storage, and plays crucial roles in lipid metabolism and cellular stress responses. In eukaryocytes, the endoplasmic reticulum (ER) accomadates neutral lipid synthesis and nascent LD biogenesis. The accumulation of neutral lipids between the leaflets of ER membranes leads to a growing lens-like structure, which eventually buds into the cytosol as nascent LD. However, the details mechanism and regulation of LD biogenesis is largely unclear.
The ER consists of two distinct morphological domains: tubules and sheets. The tubular ER network is generated by reticulons(Rtns)/REEPs, which stabilize membrane curvature, and subsequently connected by dynamin-like GTPase atlastin (ATL). Converging evidence suggests that ER tubules are mechanistically linked to LD formation.
In a study published in Journal of Cell Biology, a research group led by Prof. HU Junjie from the Institute of Biophysics of the Chinese Academy of Sciences reports that ER tubule-forming proteins and septin cytoskeletons are involved in the regulation of LD biogenesis via their interactions with ER membrane protein FIT2.
In an effort to screen new regulators of ER morphology using C. elegans, Prof. Hu and his team found the deletion of FIT2 caused ER sheets expansion. Biochemical analysis revealed that FIT2 physically interacted with ER tubule-forming proteins, including Rtn4 and REEP5, and septin cytoskeletons. Deletion or depletion of these proteins caused defective LD formation in mammalian cells and in worms. FIT2-interacting proteins were up regulated during adipocyte differentiation. Importantly, FIT2, ER tubule-forming proteins and septins were transiently enriched at nascent LD formation sites, as shown by super-resolution live cell imaging.
These findings provide important insight into the early steps of LD formation. Neutral lipids in the ER accumulate with FIT2, which in turn recruits ER tubule-forming proteins to stabilize the curvature at the growing oil lens and promote lipid preparation. The outward bulging of the oil lens is reinforced by FIT2-interacting septins, serving as a handrail.
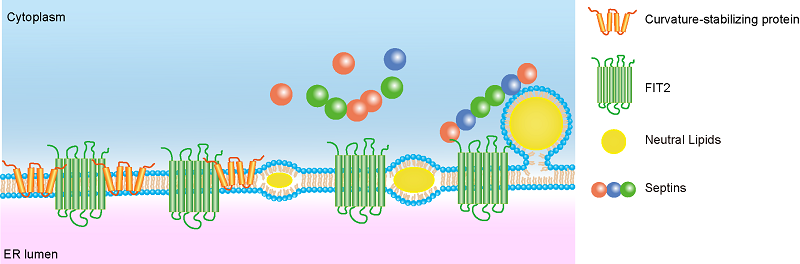
A model for FIT2-mediated nascent LD biogenesis.
Full text link: https://rupress.org/jcb/article/220/5/e201907183/211999/FIT2-organizes-lipid-droplet-biogenesis-with-ER?searchresult=1
Contact: HU Junjie
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: huj@ibp.ac.cn
(Reported by Dr. HU Junjie's group)

