Dual-targeting protein nanocage co-loaded with hydrophilic-hydrophobic drugs promotes synergistic anticancer chemotherapy
Combination chemotherapy exhibits remarkable advantages in clinic than that of single agent. However, effective combination chemotherapy remains a challenge in cancer therapy owing to the increased side effects. A proper nanocarrier capable of delivering different drugs in one nanoparticle will further increase the applications of combination chemotherapy in clinic. Protein-based nanocarrier, which is biocompatible and modifiable, is a promising candidate for tumor targeted drug delivery. However, limited by the hydrophilic nature of soluble protein, it has fallen short of achieving the objective to effectively carry hydrophobic drugs, not to mention to load both hydrophilic and hydrophobic drugs on one protein nanocarrier. Another challenge comes from uncontrollable drug release kinetics. Precisely controllable speed and sequence of drug release is an urgent problem to be solved by current nanocarriers. This is also the requirement of combined chemotherapy in clinical application.
On April 16, 2021, the latest research of the academician YAN Xiyun's team, titled "Bioengineered Dual-targeting Protein Nanocage for Stereoscopically Loading of Synergistic Hydrophilic/Hydrophobic Drugs to Enhance Anticancer Efficacy", was published in "Advanced Functional Materials" online. In response to the above problems, the researchers developed an amphiphilic multi-drug loading protein nanocage (Am-PNCage) with dual-tumor targeting property, which realized the effective delivery of hydrophilic-hydrophobic drugs and synergistic anticancer effect.
Through genetic engineering modification, researchers display functional peptides on the outer surface of the protein nanocage, which is generated by replacing the fifth helix of human H-ferritin (HFn) subunit with a functional motif composed of hydrophobic-hydrophilic-RGD peptides. The Am-PNCage possesses dual targeting property resulted from the intrinsic CD71 targeting ability of HFn and the integrin ɑvβ3 targeting ability of displayed RGD peptides. Through the hydrophilic drug entry channel in protein nanocage and hydrophobic peptides displayed on the outer surface, amphiphilic Epirubicin/Camptothecin are stereoscopically loaded into the inner cavity/outer protein shell respectively for one Am-PNCage, exhibiting spatiotemporally programmed cascade drug release pattern. The dual-targeted Am-PNCage promotes the loaded drugs penetrating various 3D tumor models in vitro, as well as traversing the brain blood barrier and accumulating in brain tumors in vivo. Moreover, the drug loaded Am-PNCage extends the half-life of small molecule drugs, shows reduced side effects and significantly enhanced synergistic efficacy against tumors, especially malignant and drug-resistant tumors. Thus, the Am-PNCage represents a novel promising protein nanocarrier for targeted combination chemotherapy.
The Am-PNCage delivery platform possesses following advantages: (a) As a self-assembling protein nanocarrier, Am-PNCage overcomes the drawbacks of current biodegradable nanoparticles, such as instability, uncontrollable drug release, and impenetrability of certain physiological barriers. (b) Am-PNCage combines dual-targeting, amphiphilic multiple drugs loading, spatiotemporally programmed cascade drug release into a single theranostic modality, which enable synergistic chemotherapeutic action and overcoming chemoresistance effectively. (c) The drug loading capability of Am-PNCage is higher than any of the current reported human ferritin based nanocarriers, and its therapeutic effect of EPI@Am-PNCage/CPT is also among the best. Importantly, this dual-targeted amphiphilic nanocage may also load multiple different drug pairs for broad-spectrum clinical applications, and is therefore an emerging combinatorial drug delivery modality for the treatment of cancers, especially for relapsed tumors and those resistant to common first-line therapeutic drugs.
Academician YAN Xiyun and Professor FAN Kelong from Institute of Biophysics, Chinese Academy of Sciences/CAS Engineering Laboratory for Nanozyme are the co-corresponding authors of this article, and Dr. WANG Zhuoran is the first author of this article. This work was financially supported by the National Natural Science Foundation of China, National Key Research and Development Program of China, Strategic Priority Research Program of CAS, CAS Key Research Program of Frontier Sciences, Youth Innovation Promotion Association of Chinese Academy of Sciences and China Postdoctoral Science Foundation.
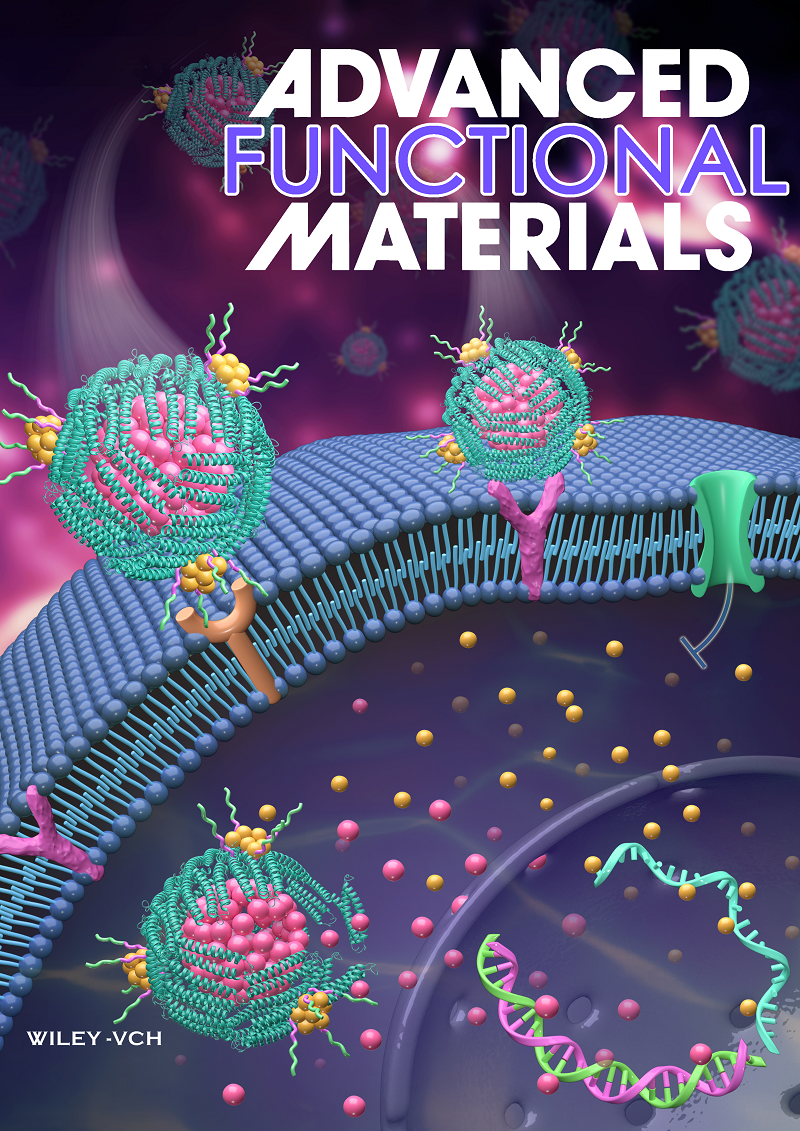
Scheme: The anticancer mechanism of dual-targeting protein nanocage co-loaded with hydrophilic-hydrophobic drugs.
Full text link: https://onlinelibrary.wiley.com/doi/10.1002/adfm.202102004
Contact: FAN Kelong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: fankelong@ibp.ac.cn
(Reported by Dr. FAN Kelong's group)

