Research reveals that Atlastin 2/3 regulate ER targeting of the ULK1 complex to initiate autophagy
Autophagy refers to a process involving the sequestration of a portion of the cytosolic constituents in a double-membrane autophagosome and its delivery to lysosomes for degradation. The core steps of autophagy include the initiation and nucleation of a cup-shaped isolation membrane (IM), and subsequent expansion and closure of the IM to form the autophagosome. The ER plays pivotal roles in multiple steps of autophagosome formation. Upon autophagy induction, the ULK1 complex is targeted to the ER to specify the autophagosome formation site, which further recruits downstream autophagy proteins to initiate IM formation. The ULK1 complex also interacts with WIPI2 to participate in establishing the contact between the ER and the IM during IM expansion into the autophagosome. Upon closure, the autophagosome disassociates from the ER and undergoes maturation steps. In mammalian cells, the ULK1 complex consists of FIP200, ULK1, Atg13 and Atg101 proteins, which dynamically interact with each other. However, little is known about how the ULK1 complex components are recruited and stabilized on the ER to specify the autophagosome formation site.
The ER transmembrane proteins ATL1, 2, and 3, a class of dynamin-like GTPases, mediate ER membrane fusion. Depletion of ATLs results in the formation of long, unbranched, and less mobile ER tubules. In this study, the researchers revealed that depletion of ATL2/3 impairs autophagy. Upon autophagy induction, diffuse cytoplasmic LC3 (LC3-I) is lipidated by PE to form LC3-II, which associates with autophagic structures. In ATL2/3 DKO cells, the ratio of LC3-II/LC3-I is dramatically decreased, levels of p62 are increased, and the formation of autophagosomes is decreased. Further studies showed that in ATL2/3 DKO cells, while the recruitment of FIP200 and ATG13 to the ER is not affected, targeting of ULK1 and ATG101 is greatly impaired. Consistent with this, the interactions of ATG13 with ULK1 and ATG101 are decreased. These results indicate that ATL2/3 contribute to the recruitment of ULK1 and ATG101 to the FIP200/ATG13-specified autophagosome formation sites on the ER. In ATL2/3 DKO cells, the interactions of WIPI2 with FIP200 and ULK1 are significantly decreased, and the percentage of the IM/autophagosome perimeter in contact with the ER is reduced, suggesting that depletion of ATL2/3 also impairs the formation of the ER-IM contact.
In summary, this research reveals that ATL2/3 interact with ATG13 and ULK1, thus contributing to the recruitment of the ULK1 complex onto the ER. ATL2/3 also participate in forming ER-IM tethering complexes. This research provides new insights into the dynamic assembly of the ULK1 complex on the ER during autophagosome formation.
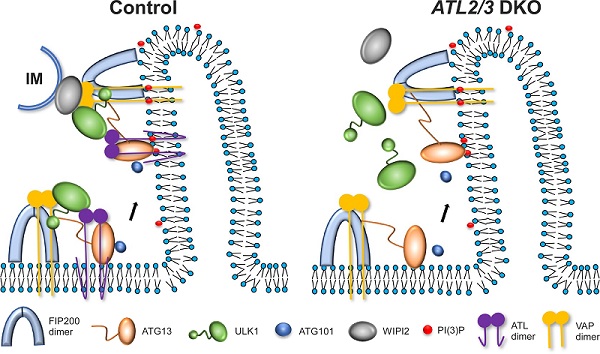
Figure. Model showing how ATL2/3 are involved in the regulation of autophagy
Professor ZHANG Hong and Professor HU Junjie from the Institute of Biophysics, Chinese Academy of Sciences/CAS are the co-corresponding authors of this article, and Dr. LIU Nan is the first author of this article. This research was supported by the National Natural Science Foundation of China, the Chinese Ministry of Science and Technology, the Beijing Municipal Science and Technology Committee, the Strategic Priority Research Program of the Chinese Academy of Sciences (CAS) and the Key Research Program of Frontier Sciences, CAS.
Full text link: https://doi.org/10.1083/jcb.202012091
Contact: ZHANG Hong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: hongzhang@ibp.ac.cn
(Reported by Dr. ZHANG Hong's group)

