Research identifies a novel regulator of cell migration and macropinocytosis
Cell polarity refers to the asymmetry observed in cell shape, structure, or localization of molecular components. Polarity can be organized spontaneously or under the guidance of extracellular biochemical and mechanical cues. Almost all types of cells exhibit some form of polarity, which enables them to carry out diverse functions. For example, in budding yeast polarity is used for directional growth and division, whereas epithelial cells display both apical-basal and planar polarity, and neurons are polarized with segregated domains of axons and dendrites specialized for receiving and transmitting signals, respectively. Considering the range of cellular processes that involve polarity, it is not surprising that misregulation of polarity is associated with developmental disorders and disease.
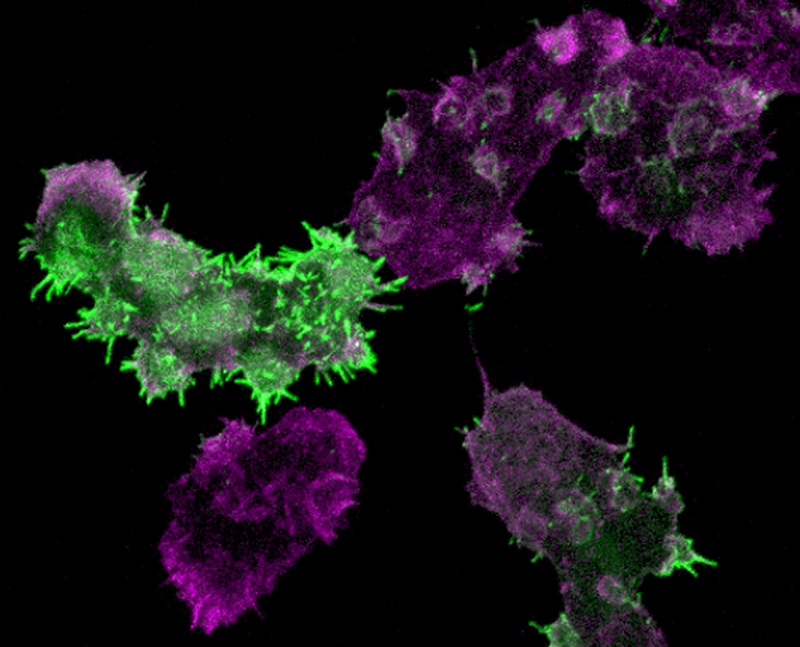
Figure 1 Leep1 overexpression alters cytoskeletal rearrangement. Cells expressing GFP-Leep1 were fixed and stained with Alexa Fluor 555-labeled phalloidin.
Establishing morphologically and functionally distinct subregions is important for cell polarity, but many aspects of this complex process are not fully understood, such as how signaling and cytoskeleton regulators are selectively targeted during different morphological states and how their activities are integrated over space and time to enable diverse cell functions. Taking advantage of the observation that both leading and trailing edge molecules respond to chemoattractant stimulation by transiently relocalizing with respect to the cell periphery, we developed a proteomics approach to comprehensively identify polarity regulators by isolating proteins exhibiting signature spatiotemporal changes following stimulation. Using this approach, we identified a leucine-rich repeat domain-containing protein we named Leep1 (leading edge enriched protein 1) as a novel leading edge-localized polarity regulator. We combined imaging, biochemical and phenotypic analyses to demonstrate that Leep1 localizes selectively at the leading edge of cells by binding to PIP3 where it modulates pseudopod and macropinocytic cup dynamics by negatively regulating the Scar/WAVE complex. The spatiotemporal coordination of PIP3 signaling, Leep1, and the Scar/WAVE complex provides a cellular mechanism for organizing protrusive structures at the leading edge. Given the importance of PIP3 and the Scar/WAVE complex in diverse cell types, our findings should pave the way for future research to further address their spatiotemporal coordination in organizing cell polarity. In addition, the proteomics approach we developed may be applied to other cell types or conditions in which polarly distributed signaling or cytoskeleton molecules exhibit synchronous dynamic changes.
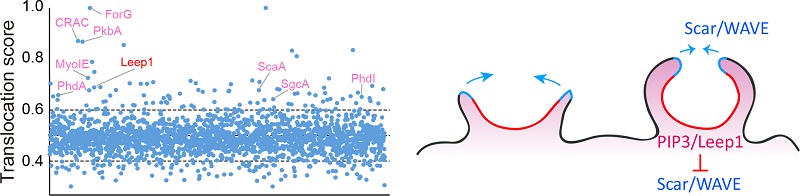
Figure 2 Leep1 interacts with PIP3 and the Scar/WAVE complex to regulate cell migration and macropinocytosis.
Professor CAI Huaqing from the Institute of Biophysics, Chinese Academy of Sciences is the corresponding author, and Dr. YANG Yihong and LI Dong are co-first authors of this article. This research was supported by the National Natural Science Foundation of China, the Chinese Ministry of Science and Technology, the Strategic Priority Research Program of Chinese Academy of Sciences.
Full text link: https://doi.org/10.1083/jcb.202010096
Contact: CAI Huaqing
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: huaqingcai@ibp.ac.cn
(Reported by Dr. CAI Huaqing's group)

