Structural Basis of MAb AA98's Inhibition on CD146-mediated Endothelial Cells
Recently, Prof. YAN Xiyun's lab, from the Institute of Biophysics in a collaboration with Prof. XIE Can from the High Magnetic Field Laboratory of the Hefei Institutes of Physical Science, reported the structural basis of mAb AA98's inhibition on CD146-mediated endothelial cells (EC) activation and designed higher affinity antibody HA98 for cancer treatment.
CD146 is an adhesion molecule that plays important roles in tumor angiogenesis, metastasis and autoimmune disease-related inflammation. Prof. YAN Xiyun's lab has been focused on the function of CD146 and the mechanism underlain, aiming to develop antibody drugs targeting CD146. Their previous studies demonstrated that CD146 triggered the signaling cascade via dimerization induced by various ligands. AA98, a monoclonal antibody (mAb) binding to CD146, shows inhibitory effects on tumor growth. However, the structural basis of CD146 activation and AA98 inhibition remains to be addressed.
In this research, the researchers described a crystal structure of the CD146/AA98 Fab complex. Structural analysis elucidated AA98 stabilized CD146 in monomer conformation thus inhibited EC activation. A higher-affinity AA98 variant (named HA98) was then rationally designed based on the complex structure in this study. Further experiments on animal model with HA98 revealed superior inhibitory effects on tumor growth to those of AA98, which suggested future applications of this antibody in cancer therapy.
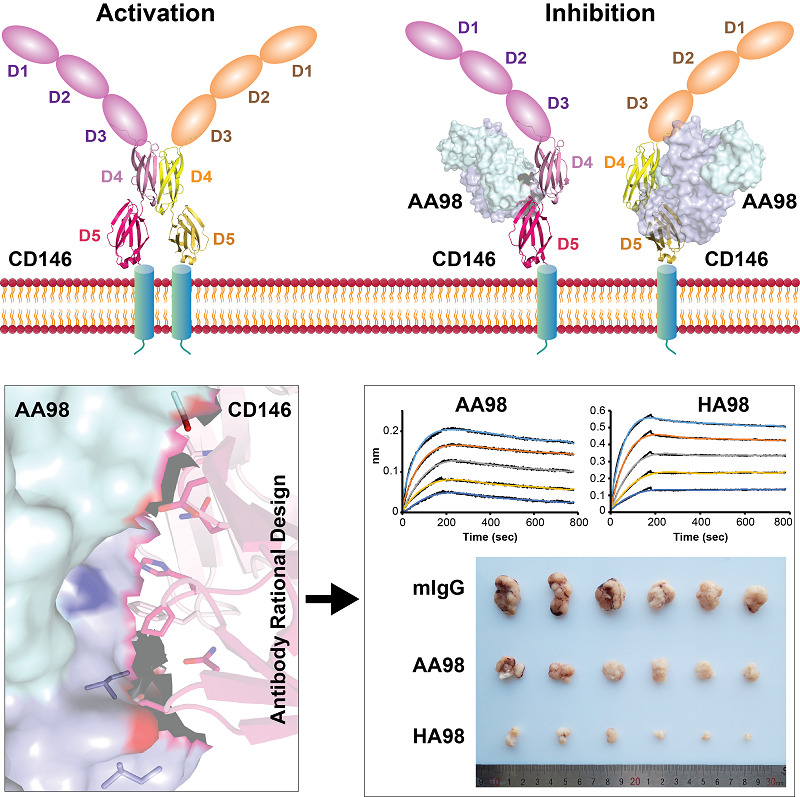
The structural basis of the inhibition of mAb AA98 on CD146 dimerizaiton and the optimization of antibody affinity with rational design
The elucidation of the structural mechanism of CD146 activation and the inhibition of AA98 on CD146 lays a structural foundation for drug design targeting CD146. Moreover, the mechanism of CD146 activation could be used for reference to clarify the function of the other IgSF adhesion molecules mediating signaling as cell membrane receptors.
This article was published in iScience. Dr. FENG Jing, Prof. YAN Xiyun from the Institute of Biophysics and Prof. XIE Can from the High Magnetic Field Laboratory of the Hefei Institutes of Physical Science, Chinese Academy of sciences are the co-corresponding authors of this paper. Dr. CHEN Xuehui and YAN Huiwen from the Institute of Biophysics contributed equally as the first authors. The research was supported by National Natural Science Foundation of China and Beijing Municipal Natural Science Foundation, China.
Fulltext link: https://www.cell.com/iscience/fulltext/S2589-0042(21)00385-0
Contact: FENG Jing
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: fengjing@ibp.ac.cn
(Reported by Dr. YAN Xiyun's group)

