Professor WANG Jiangyun's research group and collaborators made the new progress in the study of the phosphorylation barcode mechanism of G protein coupled receptor
Recently, Prof. WANG Jiangyun from Institute of Biophysics of Chinese Academy of Sciences, Prof. YU Xiao and Prof. SUN Jinpeng from School of Basic Medical Sciences of Shandong University and Prof. JIN Changwen from Peking University collaborated, published a research paper entitled "Structural studies of phosphorylation-dependent interactions between the V2R receptor and arrestin-2 "in Nature Communications.
G-protein coupled receptor (GPCR) is the largest family of membrane proteins in the human genome, responsible for about 80% of transmembrane signal transduction and involved in the regulation of most pathological and physiological processes in the human body. GPCR transforms extracellular stimuli into intracellular signals mainly through G protein and Arrestin. Before GPCRs recruit Arrestin, they are usually phosphorylated by GPCR kinases (GRKs), which produce different phosphorylation patterns and perform different functions by interaction with Arrestin. The research team of Professor SUN Jin-Peng and Professor WANG Jiangyun carried out a series of researches on the phosphorylation coding mechanism of the interaction between receptor and Arrestin, discovered the phosphorylation barcode mechanism of GPCR, and innovatively proposed the "flute model" theory of receptor phosphorylation (Nat Commun 6, 8202 (2015)). Based on the theoretical basis of the "flute model", the team further revealed the polyproline allosteric sorting mechanism of SH3 domain protein regulated by GPCR phosphorylation barcode (Nat Chem Biol 14, 876-886 (2018)). However, how a single phosphorylation site regulates the conformation and function of Arrestin remains unclear.
Flute model and extension of phosphorylation coding mechanism of Arrestin - mediated signals
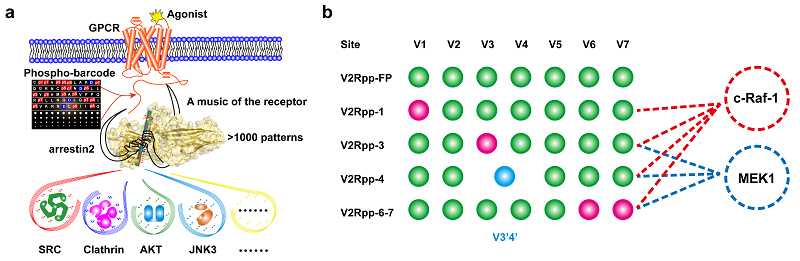
In this study, the research group used X-ray crystallography to analyze the complex structure of four different phosphorylation modes of V2R C-terminal phosphopeptide and arrestin, which directly explained the different action modes of different phosphopeptides and arrestin2. In addition, a new phosphorylation encoding method in the flute model was accidentally discovered in our work, and a new phosphate binding site " V3'4' " was found on arrestin. These results suggest that the binding pattern of Arrestin to receptor phosphorylation barcode may be more complex than previously thought, and that phosphorylation site binding at some sites determines whether other sites can be bound, and that there may be new phosphorylation coding binding sites that have not been found. At the same time, using the recently developed DeSiPher technique (Nat Commun 11, 4857 (2020)), the group found that the GPCR single phosphorylation site defect can induce specific dynamic conformational changes in the interaction region between arrestin and MEK and c-Raf-1. This suggests that a single phosphorylation site defect in GPCR can directly affect the configuration of Arrestin's distal functional domain. In addition, our team used FlAsH-BRET to detect that the receptor phosphorylation pattern differences caused by the mutation of different phosphorylation modification sites of V2R C-terminal could affect the recruitment ability of Arrestin interaction with MEK and c-Raf-1. Therefore, GPCR phosphorylation causes the configuration difference of activated Arrestin, which can lead to different signal transduction of Arrestin and regulate the function of Arrestin.
In summary, the crystal structure of the complexes between Arrestin and V2R C-terminal phosphopeptides with four different phosphorylation modes was analyzed, combined with DeSiPher and BRET, it was systematically demonstrated that a single GPCR phosphorylation site defect could cause different conformational changes in the remote functional positions of arrestin. It not only revealed the regulatory mechanism of GPCR single phosphorylation site on the function of Arrestin, but also found the important sequence principle in the phosphorylation coding process, which is a further important in-depth interpretation and expansion of the flute model of phosphorylation barcode proposed by our team and in the mechanism of the more in-depth discussion.
Professor WANG Jiangyun, Professor YU Xiao, Professor SUN Jinpeng and Professor JIN Changwen are co-corresponding authors. HE Qingtao, LIN Jingyu and XIAO Peng from School of Basic Medical Sciences of Shandong University, HUANG Shenming and JIA Yingli from Peking University, and ZHU Zhongliang Zhu from University of Science and Technology of China are co-first authors of this paper.
This research has been funded and supported by the National Key R&D Program of China, the National Science Foundation for Distinguished Young Scholars, the National Science Foundation for Excellent Young Scholars and the National Natural Science Foundation of China.
The link of this paper: https://www.nature.com/articles/s41467-021-22731-x
Contact: WANG Jiangyun
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: jwang@ibp.ac.cn
(Reported by Dr. WANG Jiangyun's group)

