Scientists identified the key portal endothelial cells controlling thymic egress
On July 1st, 2021, a collaborative team led by Dr. ZHU Mingzhao and Dr. WANG Xiaoqun from Institute of Biophysics, Chinese Academy of Sciences, published a research article entitled "Thymic Egress Is Regulated by T Cell-Derived LTβR Signal and via Distinct Thymic Portal Endothelial Cells" in Frontiers in Immunology. This study identified for the first time the key portal endothelial cells controlling thymic egress of mature thymocytes, and revealed the underlying molecular and cellular mechanisms.
The thymus is the critical lymphoid organ for the development of T cell adaptive immunity. Thymic homing of hematopoietic progenitor cells and egress of mature thymocytes are two fundamental cell trafficking behaviors, believed to take place both at a special structure called "perivascular space". However, the cellular basis of these two behaviors are not clear, and how the seemingly two different, even opposite cell trafficking behaviors could both take place at the same structure remains intriguing.
In 2016, Dr. ZHU Mingzhao's group identified a population of thymic endothelial cells that controls thymic homing of hematopoietic progenitor cells, which was named thymic portal endothelial cells, TPEC. In the current study, the researchers found that TPEC is also the site where thymic mature thymocytes egress. Using scRNA-seq analysis, the researchers found that the TPEC population is actually a heterogenous one that includes two clusters, C2 with high BST-1 expression and C6 with low or negative BST-1 expression. Using in vivo mouse models, the researchers confirmed that C2 subset of TPEC is where thymic mature thymocyte egress takes place, while C6 is where thymic homing of hematopoietic progenitor cells takes place. Thus, together with their previous study in 2016, the research group has identified two distinct thymic portal endothelial cells, eTPEC (egress TPEC) for thymic egress, and iTPEC (immigration TPEC) for thymic homing.
In this study, the researchers also revealed the detailed cellular and molecular mechanisms controlling thymic egress and eTPEC development. Using various conventional and conditional gene deficient mouse models, the researchers found that T cell-derived ligands lymphotoxin (LT) and LIGHT, and endothelial cell-derived LTβR signaling pathway are required for thymic egress, suggesting a crosstalk between T cells and endothelial cells for thymic egress control. Deficiency of LT/LIGHT-LTβR signaling axis or lack of positively selected T cells results in impaired eTPEC development. Given the important role of thymic egress for the establishment of peripheral T cell compartment, this study may provide new strategies to enhance T cell regeneration during aging or thymic injury.
Dr. XIA Huan and Dr. ZHONG Suijuan are co-first authors of this study. Dr. ZHU Mingzhao and Dr. WANG Xiaoqun are co-corresponding authors. This work was supported by grants from National Natural Science Foundation of China (31770959, 82025015, 31970866 and 81671537).
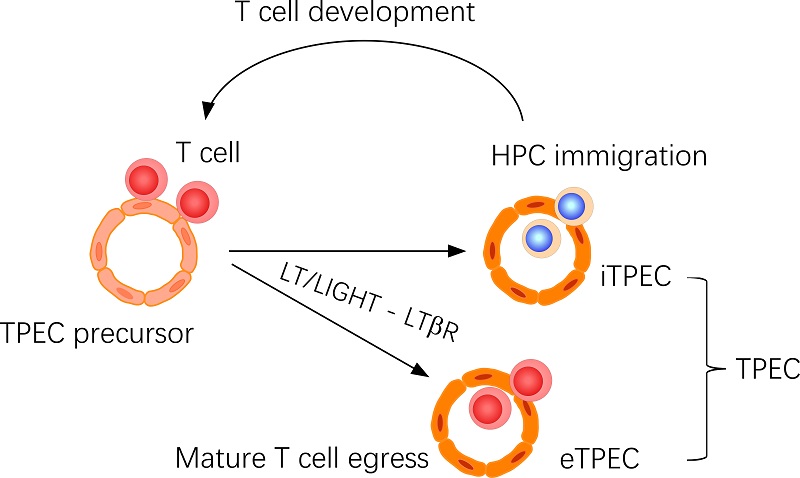
Figure. Thymic portal endothelial cells (TPECs) are associated with both thymic homing of hematopoietic progenitor cells (HPCs) and thymic egress of mature thymocytes. Current study suggests that TPECs are actually composed of two subsets, iTPECs and eTPECs, associated with HPC immigration and mature thymocyte egress, respectively.
Related links:
https://www.frontiersin.org/articles/10.3389/fimmu.2021.707404/full
http://www.nature.com/ncomms/2016/160805/ncomms12369/full/ncomms12369.html
Contact: ZHU Mingzhao
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: zhumz@ibp.ac.cn
(Reported by Dr. ZHU Mingzhao's group)

