Scientists reveal the regulatory mechanism of a secretory pathway kinase
The first evidence of protein phosphorylation was in 1883, when the secreted protein casein was shown to contain stoichiometric amounts of phosphate. Until 2012, Fam20C was identified to be the bona fide "Golgi casein kinase", which phosphorylates numerous secretory proteins. Recently, it has been reported that Fam20C participates in a wide range of biological processes, such as biomineralization, cell adhesion and migration, prohormone processing, lipid homeostasis, protein trafficking, and endoplasmic reticulum (ER) homeostasis. However, little is known about the regulatory mechanisms of Fam20C transport and maturation.
On August 5, 2021, scientists at the Institute of Biophysics, Chinese Academy of Sciences (CAS), published a research article in Proceedings of the National Academy of Sciences U S A entitled "Proteolytic processing of secretory pathway kinase Fam20C by site-1 protease promotes biomineralization". In this study, the researchers identified Fam20C as a type Ⅱ transmembrane protein with its N-terminal transmembrane (TM) domain integrated into the membrane, but not a luminal protein as previously considered. They further demonstrated that the ER-to-Golgi anterograde transport of Fam20C is mediated by COPⅡ vesicles and that the TM domain acts as a Golgi retention signal. They provided evidence that cleavage of Fam20C propeptide is catalyzed by the Golgi-resident site-1 protease (S1P), and this processing increases the secretion and kinase activity of Fam20C. By bioinformatics analysis, it was predicted that membrane spanning might be a general property for many secretory pathway kinases. Thus, this study paves the way for future studies of the secretory pathway kinase family.
Loss-of-function FAM20C mutations cause a rare and often lethal osteosclerotic bone dysplasia called Raine syndrome. S1P has also been reported to be essential for bone development, though the molecular mechanism is not clear. In this study, the researchers provided evidence that mature fully active Fam20C generated by S1P cleavage efficiently phosphorylates extracellular matrix proteins (such as osteopontin) to promote osteoblast differentiation and biomineralization. Thus, this new finding expands the physiological roles of S1P as a critical protease in regulating cholesterol homeostasis, ER stress responses, and lysosome biogenesis.
Professor WANG Lei from National Laboratory of Biomacromolecules, Institute of Biophysics, CAS is the corresponding author of the article. PhD students CHEN Xinxin and ZHANG Jiachao from Institute of Biophysics, CAS are the co-first authors. Professors XIAO Junyu (Peking University), HU Junjie and ZHANG Hong (Institute of Biophysics, CAS) make significant contributions to the work. The research was supported by the Strategic Priority Research Program of CAS; the National Natural Science Foundation of China, the National Key R&D Program of China, and the Youth Innovation Promotion Association, CAS.
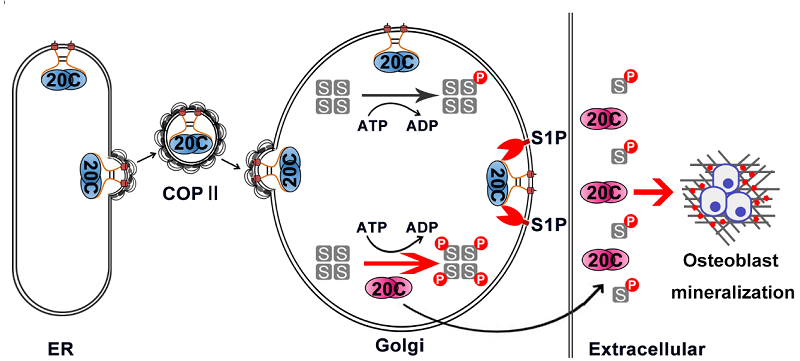
Figure: Proteolytic processing of secretory pathway kinase Fam20C by site-1 protease promotes biomineralization. 20C, Fam20C; S, substrate protein; P, phosphate group.
Full text link: https://www.pnas.org/content/118/32/e2100133118
Contact: WANG Lei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: wanglei@ibp.ac.cn
(Reported by Dr. WANG Zhizhen's group)

