The group of Prof. Sarah Perrett has made important progress in the study of mechanisms of protein liquid-liquid phase separation by single-molecule techniques
Liquid-liquid phase separation (LLPS) of proteins into biomolecular condensates has emerged as a fundamental principle underpinning cellular function and malfunction. Indeed, many human pathologies, including protein misfolding diseases, are linked to aberrant liquid-to-solid phase transitions, and disease- associated protein aggregates often nucleate through phase separation. The molecular level determinants that promote pathological phase transitions remain, however, poorly understood.
The microtubule-associated protein Tau is an intrinsically disordered protein that plays an essential role in microtubule assembly and stabilization. Full-length Tau consists of two N-terminal inserts, a proline-rich region, a microtubule-binding region (MTBR) containing four imperfect 31- or 32-residue repeated sequences, and a C-terminal region. Recently it was shown that Tau has a propensity to undergo LLPS under different conditions, and the domains involved as well as the possible biological function of LLPS have been proposed. Although these studies have shown the phase separation phenomenon of Tau both in vitro and in vivo, the interdomain conformational changes accompanying LLPS of Tau and their relationship with the self-aggregation of Tau are still not known.
In this study, we performed single-molecule Forster resonance energy transfer (smFRET) and fluorescence correlation spectroscopy (FCS) experiments to investigate the intra- and intermolecular changes of full-length Tau40 during LLPS. Using smFRET we probed interdomain conformational changes of Tau and we found that the paperclip conformation of the protein is disrupted, with the N-terminal and C-terminal domains moving apart from the MTBR domain. Strikingly, FCS experiments indicated that Tau can form intermolecular clusters, even under sub-nanomolar concentrations, which may act as nuclei for the condensation of Tau into liquid droplets with increasing protein concentration. We further demonstrated that the formation of phase separated condensates, in which the MTBR region is exposed and the protein displays increased intermolecular interactions, facilitates the conversion of Tau from the dynamic LLPS state into amyloid aggregates, which is greatly promoted by disease-associated mutations such as P301L and P301S. These results shed light on the interplay of Tau phase separation and underlying conformational changes and their relationship with conversion to the disease-associated amyloid state.
This study entitled "Conformational expansion of Tau in condensates promotes irreversible aggregation" was published on-line in the Journal of the American Chemical Society on 10 August 2021. Prof. Sarah Perrett and Dr. WU Si, both from the Institute of Biophysics, CAS, are the corresponding authors. The work was funded by the National Natural Science Foundation of China and the Ministry of Science and Technology of China.
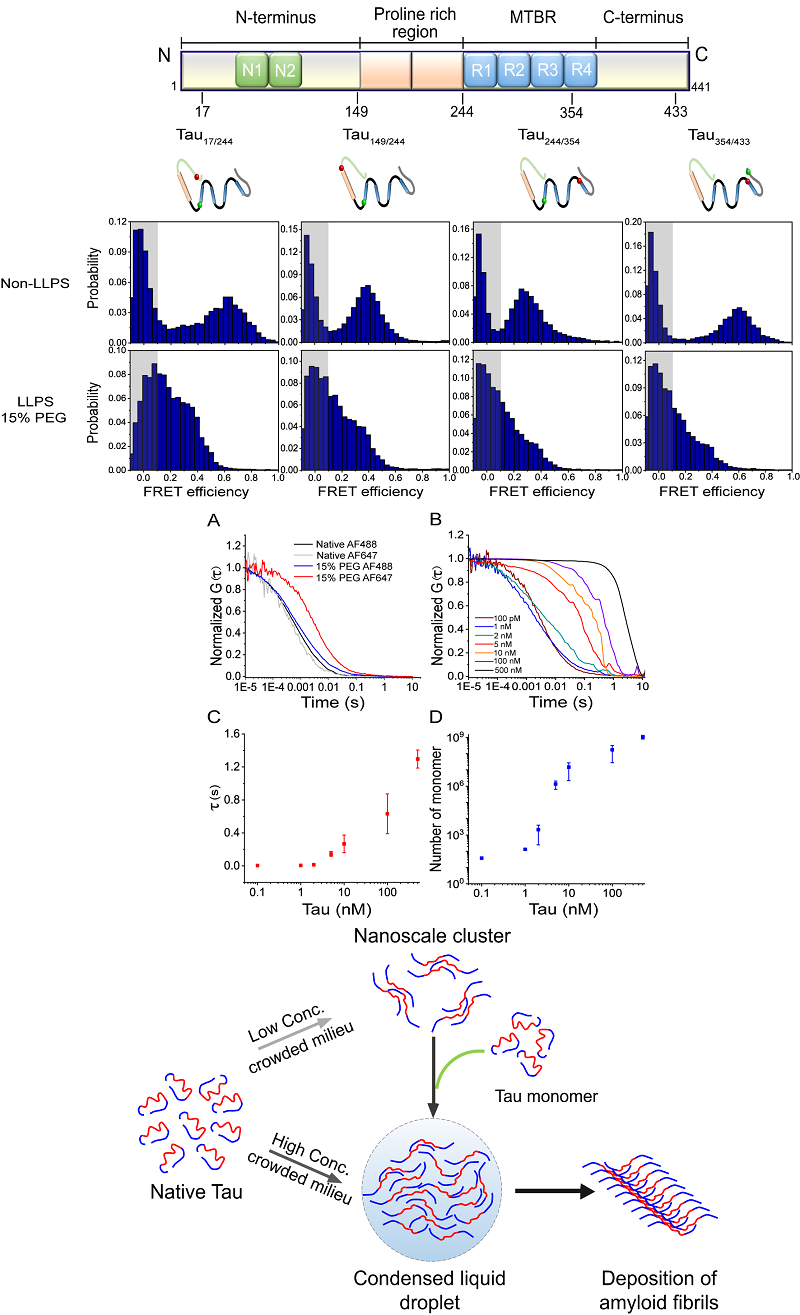
Figure: Single-molecule FRET and FCS study of the mechanism of Tau phase separation and the relationship with irreversible amyloid aggregation.
Article link: https://pubs.acs.org/doi/abs/10.1021/jacs.1c03078
Contact: Sarah Perrett, WU Si
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: sperrett@ibp.ac.cn, wusi@ibp.ac.cn
(Reported by Dr. Sarah Perrett's group)

