Scientists Uncover Modulation mechanism of Kainite Receptor by NETO2
Glutamate receptors are arguably the most important neurotransmitter-gated ion channels in our central nervous system, contributing to nearly all aspects of brain development and functions. The kainate subtypes of glutamate receptors, in particular, are not only implicated in fundamental behaviors, such as learning, memory, and sensory transduction, but they are also implicated in many neurological diseases and disorders, and become targets of several therapeutic agents.
Distinct from other two major classes of glutamate receptors - NMDA and AMPA receptors, kainate receptors (KARs) are determined to be present on both presynaptic and postsynaptic cells, and play vital roles in regulating neurotransmitter releasing and mediating neurotransmission signaling.
NETO2 is a bona fide auxiliary protein of native KARs, and profoundly modulates receptor kinetics. Even though the structures of homomeric and heteromeric KARs have been determined recently, researchers still do not understand the structure, assembly, and stoichiometry of the KARs-NETO2 complex, an understanding that is central to the interpretation of receptor physiology, pharmacology, and biophysics.
In the study published in Nature, a joint team led by Profs. ZHAO Yan and ZHANG Xuejun Cai from the Institute of Institute of Biophysics of Chinese Academy of Sciences, and Prof. SHI Yun Stone from Nanjing University recently reported an ensemble of structures of the paradigmatic rat GluK2 receptor in complex with NETO2 at antagonist DNQX-bound inhibited state and agonist kainate-bound desensitized state, illuminating, for the first time, the complete pore profile of KARs.
Cryo-EM structure of inhibited GluK2-NETO2 complex elucidates assembly, stoichiometry, and the interactions between GluK2 and NETO2. The amino terminal domain (ATD) was proposed to have no appreciable effect on KARs and AMPA receptor function, however, the researchers found that the ATD of GluK2 interacts with NETO2, and plays a predominant role in regulating the gating mechanisms of the receptor.
Furthermore, this study defined, also for the first time, the crucial elements contributing to the inward rectification in KARs, including the amphipathic H1 helix and an intracellular cap domain (ICD), which were formed by a M1-M2 linker of the receptor. These findings may also serve as a structural and functional paradigm for AMPA receptors and other ligand gated ion channels. To prove these points, the researchers augment their structural studies with patch-clamp electrophysiology recordings. This study also determined the structure of GluK2-NETO2 complex, stabilized in the desensitized state, which gave valuable insights on how NETO2 affects the kinetics of the desensitization of GluK2.
This study, entitled " Kainate receptor modulation by NETO2", was published online in Nature on September 22, 2021. The research was supported by the Chinese Academy of Sciences Strategic Priority Research Program, the National Key R&D Program of China, the National Natural Science Foundation of China, the Natural Science Foundation of Jiangsu Province and the Fundamental Research Funds for the Central Universities.
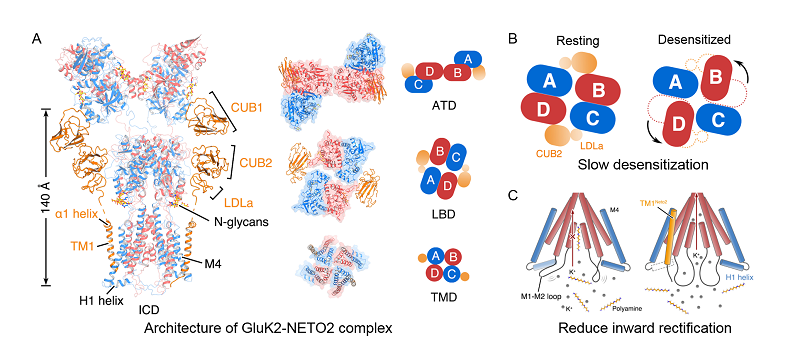
Architectures of the GluK2-NETO2 complexes (adopted from He et al., Nature)
Article link: https://www.nature.com/articles/s41586-021-03936-y
Contact: ZHAO Yan
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: zhaoy@ibp.ac.cn
(Reported by Dr. ZHAO Yan's group)

