The new cryo-electron microscope support film technology developed for high-resolution single particle analysis
As the breakthroughs in hardware and image processing algorithms, single particle analysis (SPA) using cryo-electron microscopy (cryo-EM) has become a powerful technique to study the high-resolution three-dimensional structures of biological macromolecules in solution. More and more protein structures were resolved at the near-atomic resolution with cryo-EM SPA. The first important step in this technique, which is also the key one, is cryo sample preparation. Biological macromolecules are rapidly frozen in vitreous ice for further cryo-EM imaging. However, people often encounter problems during this process, such as nonuniform/poor distribution of protein particles in the holes, serious preferred orientation, protein denaturation, etc. All these obstacles make it very hard for many samples to obtain high-resolution structures, and become the key bottlenecks in cryo-EM SPA.
Recent studies showed that the air-water interface (AWI) in the traditional cryo-EM sample preparation process leads to the above problems, and several research groups have tried multiple methods to reduce AWI effect. For example, ZHANG Xinzheng's team conducted an in-depth discussion on the AWI mechanism (J.S.B.,Vol. 213, 107783); SUN Fei's group and their colleagues developed the GiG (ZL201310489148.1) and ANTA film (CN201810326897.5, Progress in Biophysics and Molecular Biology 160, 5-15), which has been widely used in cryo-EM field of China. Based on these studies, SUN Fei's team collaborated with QIAO Mingqiang's team to develop a new cryo-EM support material named HFBI-film, which can effectively solve those problems caused by the AWI effect. This technology improves the flux and efficiency of high-resolution SPA for biological macromolecular complexes. We believe that this new technique will be an effective solution for problems in cryo-EM sample preparation.
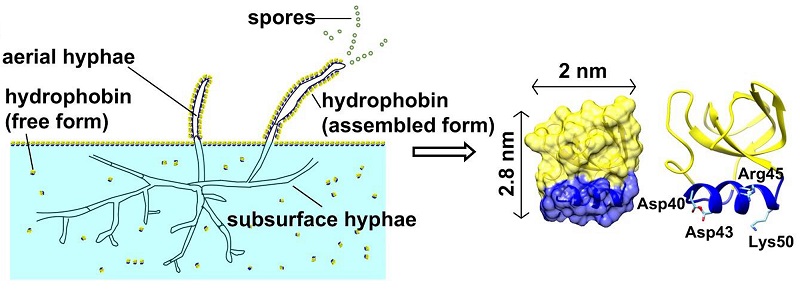
Figure 1. Schematic diagram of physiological function of the fungal hydrophobin HFBI and its three-dimensional structure
Hydrophobins are widely used in emulsions, drug delivery, biomaterial modification and biosensor researches due to the good biocompatibility, unique self-assembly characteristics and high film forming stability. Hydrophobin HFBI, a class of type II hydrophobins produced by Trichoderma reesei, can self-assemble into a highly ordered two-dimensional crystal monolayer film at the AWI (Figure 1). In this study, the continuous two-dimensional crystal film formed by self-assembly of hydrophobin HFBI were used to cover the holey amorphous nickel-titanium alloy foil grid (Figure 2) for cryo-EM sample preparation. The HFBI film-covered grid is naturally hydrophilic and doesn't need to do plasma cleaning. The problem of AWI can be overcome by absorbing protein particles onto the HFBI film through electrostatic interactions. In addition, more uniform and thinner ice with low background contrast can be formed, which is conducive to cryo-EM imaging with high resolution and high signal-to-noise ratio. The HFBI film covered-grids were used to successfully resolve the high resolution cryo-EM structures of six proteins (Figure 3), including two protein samples with obvious preferred orientation phenomenon in previous cryo-EM experiments: catalase and influenza hemagglutinin (HA) trimer. The structure of a small protein (64 kDa) was also resolved to near-atomic resolution. These results indicate that the new type of cryo-EM grid can effectively solve the AWI problem and be widely applied to samples with different molecular weights. In addition, the researchers proved that the hydrophobin membrane adsorbed protein particles through electrostatic interactions. This interaction can be changed by adjusting the pH value of the sample solution, thus providing a clear direction and means for the future use of the HFBI film covered-grid.
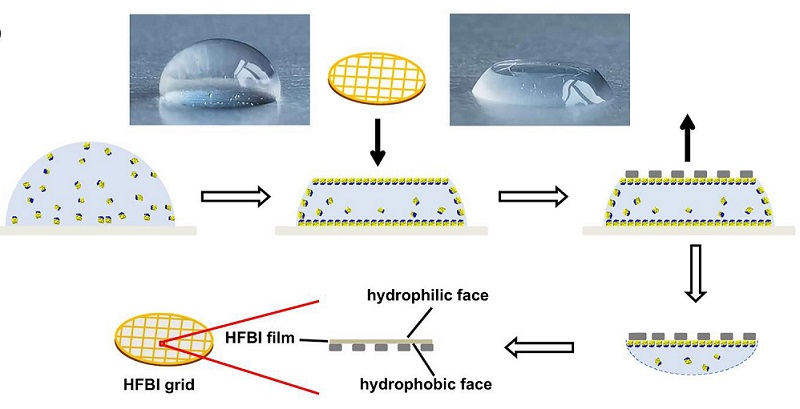
Figure 2. Scheme of preparing an HFBI film-covered ANTA foil grid.

Figure 3. A plot showing the relationship between the molecular weight and the final resolution achieved in this study using the HFBI film.
This study was completed by the cooperation of Institute of Biophysics (IBP), Chinese Academy of Sciences (CAS) and Nankai University. The co-corresponding authors are Prof. SUN Fei from IBP and Prof. QIAO Mingqiang from Nankai. FAN Hongcheng, PhD student of IBP, designed and prepared the HFBI film covered grid, and was the first author of this work. All cryo-EM sample preparation and data collection work were performed at the Center for Biological Imaging (CBI) in IBP. This work was supported by the Ministry of Science and Technology of China, the Sino-Swiss Scientific and Technological Cooperation Project by the Ministry of Science and Technology of China, the National Natural Science Foundation of China, the Chinese Academy of Sciences Strategic Priority Research Program and the Beijing Municipal Science and Technology Commission.
The production of HFBI film-covered grids has been submitted as a Chinese patent of invention (belonging to H.F., F.S., B.W., M.Q., and Y. Z.) with the application number CN202110576212.4.
Article link: https://doi.org/10.1038/s41467-021-27596-8
Contact: SUN Fei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: feisun@ibp.ac.cn
(Reported by Dr. SUN Fei's group)

