The extracellular Ero1α/PDI electron transport system regulates platelet function
Platelets are the second largest number of cells in the blood. The primary physiological role of platelets is to sense vascular injury, adhere and accumulate at the site of injury, seal the wound and prevent the loss of blood. However, excessive and abnormal activation of platelet results in severe cardiovascular diseases, including myocardial infraction and stroke, which are the leading cause of death globally. Hence, it is of special significance to unravel the mechanism of platelet aggregation and thrombosis. Emerging evidences have shown that protein disulfide isomerase (PDI) plays a critical role in the regulation of platelet aggregation and thrombosis. The inhibition of PDI is being evaluated clinically as a novel antithrombotic strategy.
PDI is an endoplasmic reticulum (ER)-resident oxidoreductase that catalyzes the formation of correct disulfide bonds during protein folding. ER oxidoreductin-1α (Ero1α) and PDI constitute the pivotal oxidative folding pathway in the ER. PDI possesses two vicinal cysteines in the -Cys-Gly-His-Cys- motif that either form a disulfide bond (S-S) or exist in a sulfhydryl form (-SH), forming oxidized or reduced PDI, respectively. The redox states of functional PDI during the regulation of platelet aggregation, however, remains to be elucidated. Furthermore, whether Ero1α and PDI constitute an extracellular electron transport pathway to mediate platelet aggregation is an open question.
On January 20, 2022, Professor WANG Chih-chen/WANG Lei's group at the Institute of Biophysics, Chinese Academy of Science (CAS), published a research article in Redox Biology entitled "The extracellular Ero1α/PDI electron transport system regulates platelet function by increasing glutathione reduction potential". This study unravels that the Ero1α/PDI electron transport system plays an important role in regulating platelet aggregation and provides mechanistic explanation for this relevant phenomenon.
The researchers found that oxidized but not reduced PDI promoted platelet aggregation and adhesion mediated by the integrin αIIbβ3. With reconstituted Ero1α/PDI oxidative system, the researcher found that reduced PDI enhanced platelet aggregation in combination with WT Ero1α that can oxidize it. The inhibition of platelet endogenous Ero1α resulted in a substantial increase of reduced PDI without changing total thiols on platelet surface, suggesting that platelet Ero1α specifically oxidized PDI on platelet surface. The platelet surface Ero1α/PDI system oxidized GSH and established an GSH/GSSG reduction potential optimal for platelet aggregation. Finally, inhibitor targeting the b' domain can efficiently disrupt the Ero1α/PDI electron transport system, proposing that targeting the functional interplay between PDI and Ero1α may be a new strategy for antithrombotic therapy.
Professor WANG Lei from National Laboratory of Biomacromolecules, Institute of Biophysics, CAS is the corresponding author of the article, and Dr. WANG Lu is the first author and the co-corresponding author. The study was supported by the National Natural Science Foundation of China; the National Key R&D Program of China; the Strategic Priority Research Program of CAS; the Youth Innovation Promotion Association, CAS and the China Postdoctoral Science Foundation.
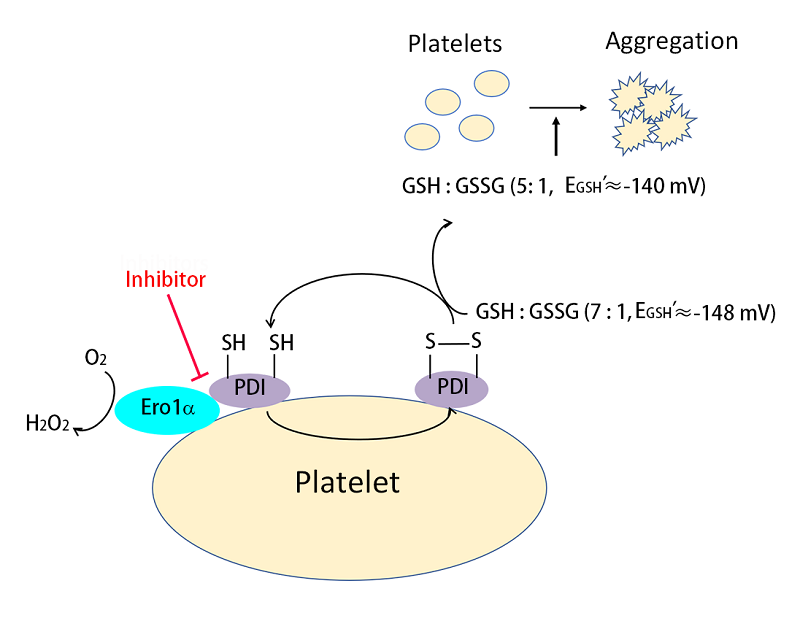
Working model of the Ero1α/PDI electron transport system on platelet aggregation.
Article link: https://www.sciencedirect.com/science/article/pii/S2213231722000167?via%3Dihub
Contact: WANG Lei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: wanglei@ibp.ac.cn
(Reported by Dr. WANG Chih-chen/Wang Lei's group)

