CD146 Associates with Gp130 to Control a Macrophage Pro-inflammatory Program that Regulates the Metabolic Response to Obesity
Obesity and its associated comorbidities, type 2 diabetes and cardiovascular disease are life-threatening chronic diseases. Compelling evidence suggests a causal role of chronic inflammation in obesity-associated pathologic conditions, including liver damage and insulin resistance. Adipose tissue macrophages (ATMs), constituting about 5% of adipose stromal vascular cells or fractions (SVFs) in the lean state and up to 50% of SVFs during obesity, have been recognized as key contributors to chronic inflammation.
Macrophages are frequently classified as pro-inflammatory, M1 or 'classically activated'; or anti-inflammatory, M2 or 'alternatively activated'. ATMs in lean organisms tend to express genes associated with a M2-like phenotype, whereas ATMs in obese organisms typically express genes associated with a M1-like phenotype and contribute to obesity-induced adipose tissue inflammation and associated systemic insulin resistance dysfunction. It is generally accepted that M1-polarization is induced to promote pro-inflammatory changes in adipose tissue, which may lead to systemic insulin resistance, while M2-polarization is associated with less inflammation, lower body weight, improved insulin sensitivity and white fat browning. Targeting adipose tissue-resident macrophages may serve as a potential anti-obesity immunotherapy.
Recently, Professor YAN Xiyun's group at the Institute of Biophysics, Chinese Academy of Sciences, reported that CD146+ ATMs accumulate in adipose tissue during diet-induced obesity and are associated with increased body weight, systemic inflammation, and obesity-induced insulin resistance. Inactivating the macrophage CD146 gene or antibody targeting of CD146 alleviates obesity-related chronic inflammation and metabolic dysfunction. Macrophage CD146 interacts with Glycoprotein 130 (Gp130), the common subunit of the receptor signaling complex for the interleukin-6 family of cytokines. CD146/Gp130 interaction promotes pro-inflammatory polarization of ATMs by activating JNK signaling and inhibiting the activation of STAT3, a transcription factor for M2-like polarization. Disruption of their interaction by anti-CD146 antibody or interleukin-6 steers ATMs toward anti-inflammatory polarization, thus attenuating obesity-induced chronic inflammation and metabolic dysfunction in mice. The results suggest that macrophage CD146 is an important determinant of pro-inflammatory polarization and plays a pivotal role in obesity-induced metabolic dysfunction. CD146 could constitute a novel therapeutic target for obesity complications.
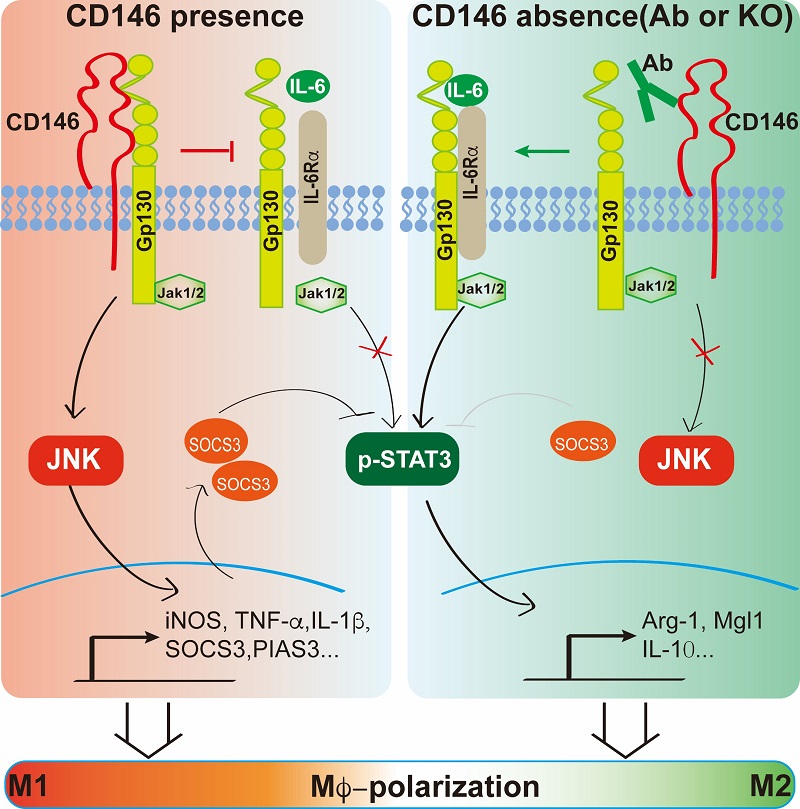
Figure 1. Proposed model for the role of the CD146-Gp130 interaction in macrophage polarization. Upon ACM stimulation, CD146 interacts with Gp130 to inhibit ACM (mainly IL-6)-induced STAT3 activation. It also activates the JNK signal and promotes the expression of M1-related genes and SOCS3, which enhances the STAT3 inhibition. Conversely, when CD146 is blocked by an antibody or deleted, its association with Gp130 is disrupted; in turn, it inhibits the JNK signaling and activates STAT3 signaling, promoting the anti-inflammatory polarization, which is similar to the effect of a larger amount of IL-6-stimulation alone.
Article link: http://doi.org/10.1002/advs.202103719
Contact: DUAN Hongxia
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: cherryshoen@ibp.ac.cn
(Reported by Dr. YAN Xiyun's group)

