Single-molecule analysis reveals distinct lipid membrane-mediated pathways of Tau assembly
The microtubule-associated protein Tau regulates the assembly and stability of microtubules in the physiologically functional state. Misfolding and amyloid aggregation of Tau protein is associated with Alzheimer's disease and other Tauopathies. Although the in vitro amyloid aggregation of Tau can be induced by polyanionic factors, the trigger for Tau aggregation in vivo is still unclear. Growing evidence indicates that lipid membranes play an important role in both functional and pathological aspects of Tau. The interaction of Tau protein with lipid membranes can enhance the ability of Tau to stabilize microtubules and assist in localisation of organelles. On the other hand, lipid membranes can accelerate amyloid formation of Tau, which causes disruption of the cellular membrane and finally leads to apoptosis. Differences in Tau-membrane interaction mechanisms that lead to physiological and pathological states remain to be explored in detail.
In this study, we performed single-molecule Forster resonance energy transfer (smFRET) and fluorescence correlation spectroscopy (FCS) experiments to investigate the intramolecular conformational changes and intermolecular assembly of full-length Tau40 in the presence of lipid vesicles formed by 1,2-dimyristoyl-sn-glycero-3-phosphatidylserine (DMPS). We found that the conformation of Tau becomes expanded by opening of the N-terminal and C-terminal domains of Tau upon binding to increasing concentrations of DMPS vesicles. Further, smFRET and FCS results indicate the formation of Tau oligomers at low DMPS concentrations. The conformational expansion and intermolecular oligomerization of Tau induced by low concentrations of DMPS vesicles greatly accelerates the fibrillation of Tau. At high DMPS concentrations, the lipid membranes bind tightly to extended Tau and this inhibits Tau aggregation. These results reveal the underlying mechanisms by which lipid membranes influence amyloid formation of Tau, providing a foundation for further understanding of the pathogenesis and physiology of the interplay between Tau protein and lipid membranes.
This study entitled "Distinct lipid membrane-mediated pathways of Tau assembly revealed by single-molecule analysis" was published on-line in the journal Nanoscale on 8 March 2022. Dr. WU Si, an Associate Professor in the group of Prof. Sarah Perrett, Institute of Biophysics, CAS, is the corresponding author. The work was funded by the National Natural Science Foundation of China and the Ministry of Science and Technology of China.
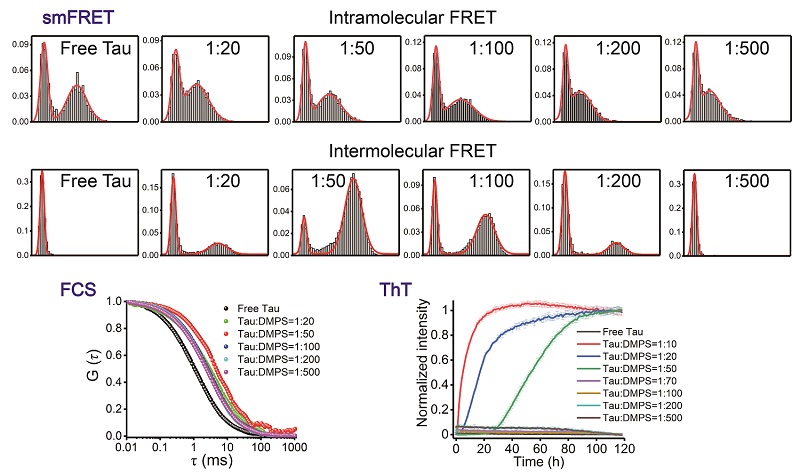
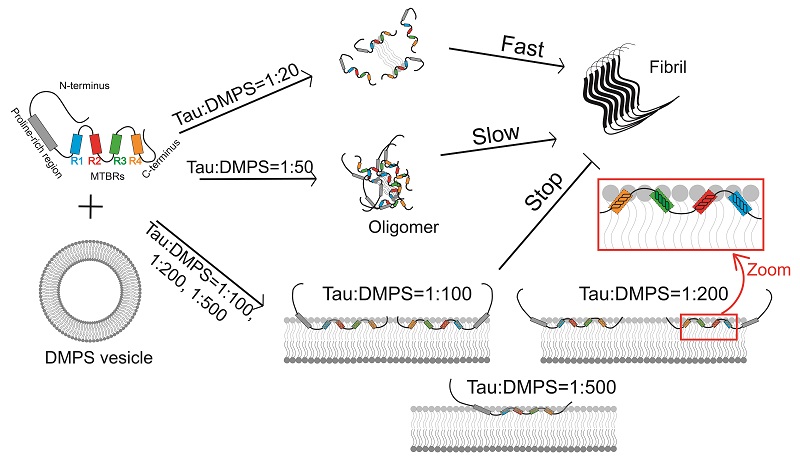
Figure 1. Single-molecule FRET and FCS reveal the effects of lipid membranes on the conformation and aggregation of Tau.
Article link: https://pubs.rsc.org/en/Content/ArticleLanding/2022/NR/D1NR05960B
CONTACT: Si Wu
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64888496
Email: wusi@ibp.ac.cn
(Reported by Dr. Sarah Perret's group)

