Research reveals mechanism of macropinosome maturation
Macropinocytosis is an evolutionarily conserved mechanism for nonspecific bulk uptake and internalization of extracellular fluid. The process has been observed in a variety of cell types. In the social amoeba Dictyostelium, macropinocytosis is the major route by which axenically grown cells obtain nutrients. In mammals, macropinocytosis plays an important role in antigen sampling, pathogen infiltration, cell migration, and regulation of synaptic activity. Recently, macropinocytosis was shown to facilitate tumor cell survival and proliferation in the nutrient-poor tumor microenvironment by engulfing extracellular proteins and fatty acids.
Macropinosomes are much larger in size than endosomes derived from microendocytic pathways, such as clathrin-mediated endocytosis. The capture and processing of large volumes of extracellular fluid poses unique challenges for the cell. Although much has been learned about the membrane lipid and cytoskeleton requirements of macropinosome formation, the molecular machinery regulating the subsequent maturation steps remains to be elucidated.
In a recent study published in Nature Communications, Professor CAI Huaqing's group at the Institute of Biophysics Chinese Academy of Sciences revealed that a Rab GAP cascade mechanism regulates macropinosome maturation. They first found that macropinosomes acquire Rab5 and Rab7 in a sequential manner after scission from the plasma membrane. By searching for proteins that localize on macropinosomes during the Rab5-to-Rab7 transition stage, they uncovered a complex composed of two proteins named PripA and TbcrA. They showed that the Rab5-to-Rab7 conversion involves fusion of Rab5-marked early macropinosomes with Rab7-marked late macropinosomes. PripA links the two membrane compartments by interacting with PI(3,4)P2 and Rab7. In addition, PripA recruits TbcrA, which acts as a GAP, to turn off Rab5. Thus, the conversion to Rab7 is linked to inactivation of the upstream Rab5. Consistently, disruption of either pripA or tbcrA impairs Rab5 inactivation and macropinocytic cargo processing.
Therefore, Rab5, Rab7, and the PripA-TbcrA complex constitute a Rab GAP cascade on the macropinosomal membranes that ensures a programmed Rab switch during macropinosome maturation and the directionality of membrane trafficking. Together with other examples in the literature, these studies indicate that Rab GAP cascade may be a general mechanism for generating a programmed switch in the Rab association on membrane compartments as they move along endocytic or exocytic pathways.
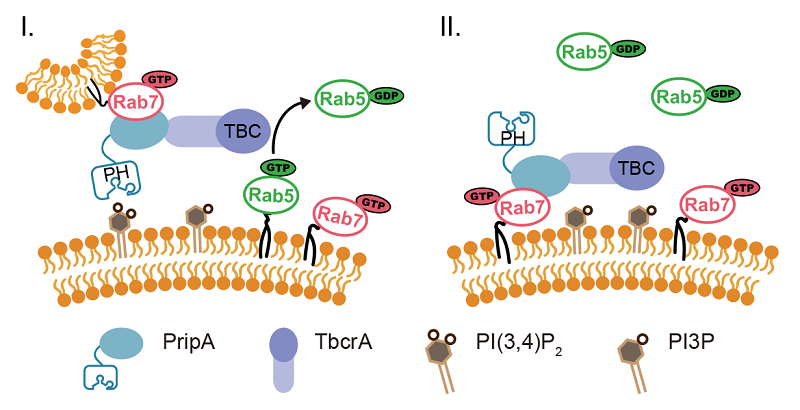
Figure. Rab5, Rab7, and the PripA-TbcrA complex constitute a Rab GAP cascade regulating macropinosome maturation.
Full text link: https://www.nature.com/articles/s41467-022-29503-1
Contact: CAI Huaqing
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: huaqingcai@ibp.ac.cn
(Reported by Dr. CAI Huaqing's group)

