Researchers reveal vesicle docking on actin cytoskeleton is critical for MVB formation
Multivesicular bodies (MVBs) are important organelles along the endocytic pathway that contain multiple lumenal vesicles. In the cells, MVBs deliver membrane proteins and cytosolic components to lysosomes for degradation or fuse with the plasma membrane to release exosomes for inter-cellular communication. Altogether, the degradative and secretory MVB pathway are essential for regulating fundamental processes such as nutrient uptake, immunity and cell signaling etc. Despite its fundamental roles in maintaining cellular homeostasis, mechanisms controlling MVB biogenesis are not clearly understood.
In a recent study published on Journal of Cell Biology, a research team led by Prof. WANG Xiaochen from the Institute of Biophysics, Chinese Academy of Science, revealed for the first time that docking of MVBs on actin cytoskeleton facilitated MVB formation.
From a forward genetic screen performed in the nematode C. elegans, they identified FLN-2, an F-actin crosslinking protein, as a novel regulator of MVB biogenesis. They found that FLN-2 closely associated with the V-ATPase-decorated MVBs and loss function of FLN-2 impaired MVB biogenesis, reducing both of MVBs and intralumenal vesicles (ILVs) number in the nematode epidermis.
Moreover, they found that FLN-2 partially overlapped with F-actin and is required for F-actin organization. Further analysis indicated that the integrity of actin cytoskeleton is necessary for MVB biogenesis, as disruption of actin cytoskeleton greatly impaired MVB formation. They also found that inactivation of V0 or V1 subunits of V-ATPase by RNAi affected MVB biogenesis, suggesting a role in regulating MVB formation.
Through super-resolution imaging, they revealed that FLN-2 may act as a bridging molecule to dock MVBs on actin platform to promote its formation. Mechanistically, they further showed that FLN-2 could directly interact with F-actin and V1-E subunit of the V-ATPase VHA-8 through its N-terminal CH domain, therefore offering the link between MVB and actin.
This study revealed that MVB docking on actin cytoskeleton via FLN-2 is important for MVB biogenesis and the novel role of FLN-2 in MVB biogenesis may shed light on the understanding of filamin-related diseases.
This work was supported by grants from the National Key R&D Program of China and the National Natural Science Foundation of China.
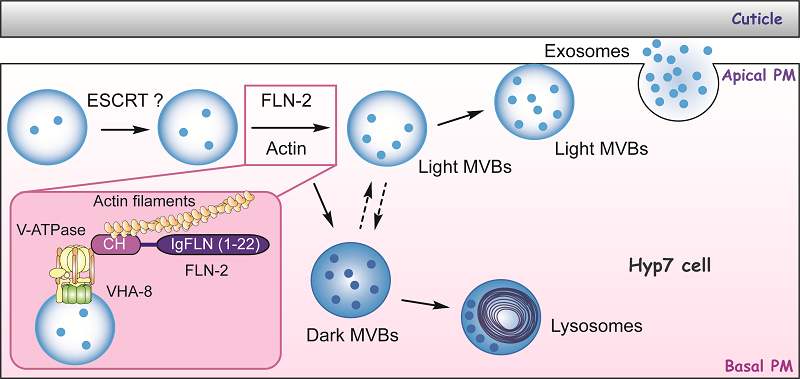
Model of FLN-2 and actin in MVB biogenesis
Article link: https://doi.org/10.1083/jcb.202201020
Contact: WANG Xiaochen
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: wangxiaochen@ibp.ac.cn
(Reported by Dr. WANG Xiaochen's group)

