Itaconate is a lysosomal inducer that promotes antibacterial innate immunity
Innate immunity is a defense process utilized by mammalian host cells to prevent the infections of pathogens, such as bacteria and viruses, degradation of which is depending on lysosome, a kind of organelle located at the cytoplasm. Therefore, elevation of the intracellular lysosomal number is able to strengthen innate immunity. Previous studies demonstrated that TFEB is a key transcription factor controlling lysosomal biogenesis. Under normal conditions, mTOR-mediated TFEB S211 (S211 in humans and S269 in mice) phosphorylation triggers the binding of 14-3-3 regulatory proteins to TFEB, leading to TFEB cytosolic retention. Under stressful conditions, such as nutrition deprivation, oxidation stress, or pathogen infections, TFEB phosphorylation is disrupted by phosphatase and/or mTOR inactivation, leading to release from the binding of 14-3-3 proteins and nuclear translocation. In the nucleus, TFEB binds to the Coordinated Lysosomal Expression and Regulation (CLEAR) element located at promoter regions of lysosomal and autophagic genes, prompting their activation and subsequently lysosomal biogenesis.
Itaconate, an immunomodulatory metabolite derived from tricarboxylic acid cycle, is converted from cis-aconitate by the enzyme immune-responsive gene 1 (IRG1) in the matrix of mitochondria, in response to LPS stimulus or bacterial/viral infections. Structurally, itaconate, as an α, β-unsaturated carboxylic acid, contains a classic electrophilic backbone, thus is able to alkylate protein cysteine residues by a thia-Michael addition reaction.
Recently, Dr. LI Xinjian's group at the Institute of Biophysics, Chinese Academy of Sciences, reports that itaconate is a lysosomal inducer that promotes antibacterial innate immunity. Mechanistically, itaconate directly alkylates human TFEB at cysteine 212 (Cys270 in mice) to induce its nuclear localization by antagonizing mTOR-mediated phosphorylation and cytosolic retention. Functionally, abrogation of itaconate synthesis by IRG1/Irg1 knockout or expression of an alkylation-deficient TFEB mutant impairs the antibacterial ability of macrophages in vitro. Furthermore, knockin mice harboring an alkylation-deficient TFEB mutant display elevated susceptibility to Salmonella typhimurium infection, whereas in vivo treatment of OI, a cell-permeable itaconate derivative, limits inflammation. Our study identifies itaconate as an endogenous metabolite that functions as a lysosomal inducer in macrophages in response to bacterial infection, implying the potential therapeutic utility of itaconate in treating human bacterial infection.
This work was supported by the National Natural Science Foundation of China; Key Program of the Chinese Academy of Sciences; the National Key R&D Program of China; and the National Science Foundation for Young Scientists of China.
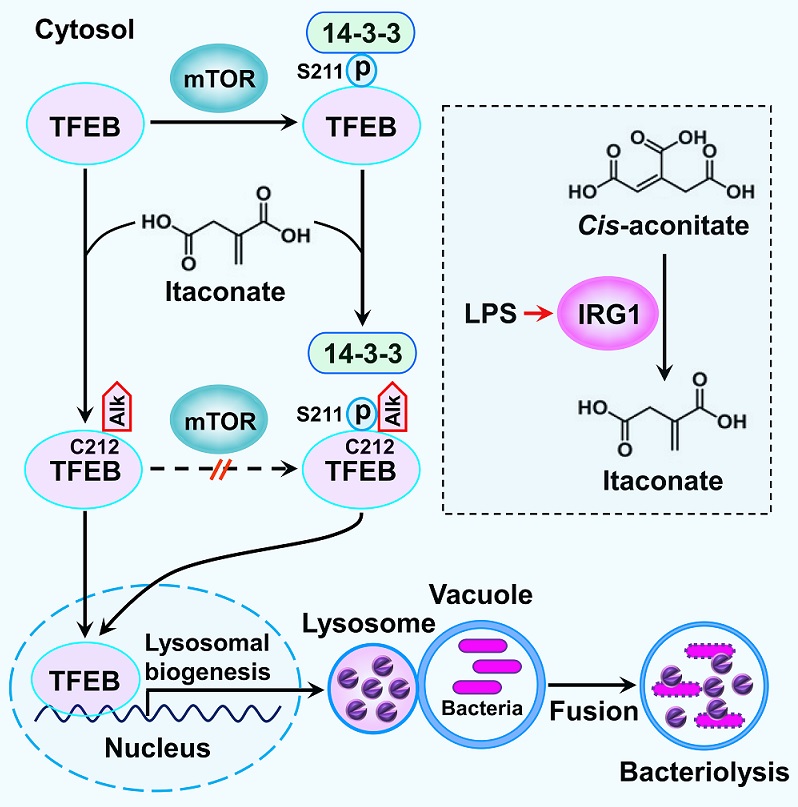
Figure. A schematic diagram shows the lysosomal inducer role of itaconate in response to bacterial infection. Itaconate, synthesized from cis-aconitate by the enzyme IRG1 upon LPS stimulation, alkylates TFEB at Cys212. TFEB alkylation disrupts the binding of 14-3-3 proteins to TFEB by blocking mTOR-dependent TFEB S211 phosphorylation or interfering with the accessibility of 14-3-3 proteins to S211-phospho-TFEB, leading to TFEB nuclear translocation. Nuclear TFEB promotes lysosomal biogenesis to facilitate bacteriolysis in macrophages. Alk, alkylation.
Article link:https://www.cell.com/molecular-cell/fulltext/S1097-2765(22)00443-9
Contact: LI Xinjian
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: lixinjian@ibp.ac.cn
(Reported by Dr. LI Xinjian's group)

