Scientists reveal how the FW domain of autophagy receptor Nbr1 recognizes protein
On June 25, 2021, Dr. YE Keqiong's team at the Institute of Biophysics (IBP), Chinese Academy of Sciences and Dr. DU Li-Lin's team at the National Institute of Biological Sciences (NIBS), Beijing, published a collaborative study in Nature Communications entitled "Structural mechanism of protein recognition by the FW domain of autophagy receptor Nbr1". The study discovered how the four-tryptophan (FW) domain of autophagy receptor Nbr1 structurally recognizes a cargo protein.
Autophagy transports cytoplasmic materials to lysosomes and is important for cell homeostasis. Neighbor of BRCA1 (Nbr1) is a conserved autophagy receptor that specifically recognizes cargos in selective autophagy. Nbr1 proteins from different organisms contain a variable number of domains, but share a signature FW domain whose function remains unclear.
The newly published study found that Nbr1 from the filamentous fungus Chaetomium thermophilum uses its FW domain to bind the α-mannosidase Ams1 and delivers Ams1 to the vacuole by conventional autophagy in heterologous fission yeast. The structure of the Ams1-FW complex was determined at 2.2 Å resolution by cryo-electron microscopy. The FW domain simultaneously binds two subunits of Ams1 tetramer, hence recognizing its quaternary structure. The structure shows that the N-terminal di-glycine of Ams1 is specifically recognized by a conserved pocket of the FW domain. The peptide-binding pocket of the FW domain becomes degenerated in fission yeast Nbr1, which binds Ams1 with a ZZ domain instead. This study illustrates for the first time the protein binding mode of the FW domain and also reveals the versatility of Nbr1-mediated cargo recognition.
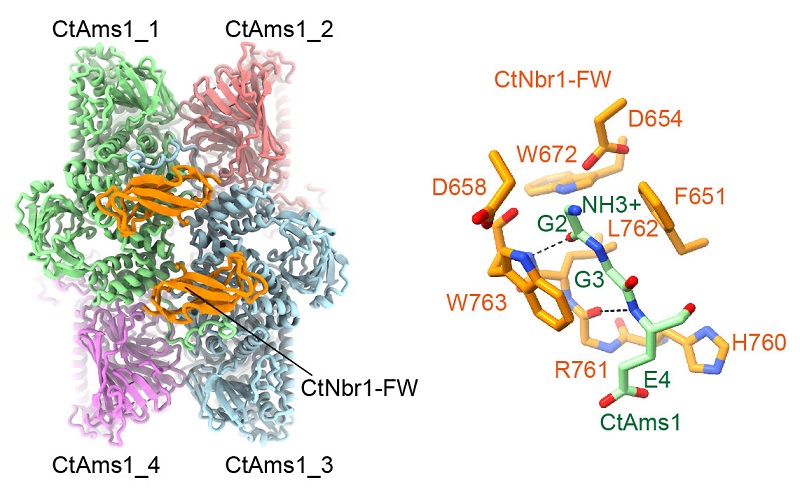
Figure. Cryo-EM structure of Ams1-Nbr1 FW complex
In the collaborative study, Prof. YE Keqiong at IBP and Prof. DU Li-Lin at NIBS were the corresponding authors. Dr. ZHANG Jianxiu at IBP and Drs. WANG Ying-Ying and PAN Zhao-Qian at NIBS were the co-first authors. The research was supported by National Natural Science Foundation of China, Strategic Priority Research Program of Chinese Academy of Sciences, National Key R&D Program of China, Chinese Ministry of Science and Technology and the Beijing municipal Government.
Full text link: https://doi.org/10.1038/s41467-022-31439-5
Contact: Ye Keqiong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: yekeqiong@ibp.ac.cn
(Reported by Dr. Ye Keqiong's group)

