SARS-CoV-2 ORF8 reshapes the ER through forming mixed disulfides with ER oxidoreductases
SARS-CoV-2 pandemic has become a major threat to the global public health. Currently, there is a lack of understanding how viral infection affects host cell life. The endoplasmic reticulum (ER) is the site for the folding and post-translational modification of secretory and membrane proteins in eukaryotic cells, and is also important for assembly and maturation of coronaviruses. Viruses hijack host cells to synthesize their own proteins, thereby increasing ER burden and causing ER stress. Elucidating the molecular mechanism of SARS-CoV-2 induced ER stress is vital for our understanding of the pathogenesis and high infectivity of the virus, and is also helpful for exploring the potential intervention and treatment for COVID-19.
On 28th June, 2022, Prof. WANG Chih-chen/WANG Lei's group at the Institute of Biophysics, Chinese Academy of Science (CAS), published a research article in Redox Biology entitled "SARS-CoV-2 ORF8 reshapes the ER through forming mixed disulfides with ER oxidoreductases". This study revealed SARS-CoV-2 accessory protein ORF8 escapes the degradation system by forming mixed disulfide complexes with ER oxidoreductases and accumulates in the ER lumen, thereby causing ER stress in host cells.
SARS-CoV-2 ORF8 is the least conserved protein in coronaviruses with only 31% sequence similarity to SARS-CoV-1 ORF8ab. The researchers first determined that ORF8 is located in the ER lumen, and found that it preferentially forms intermolecular disulfide bonds with ER oxidoreductases, such as PDI and ERp44, in the ORF8 interactome. Thus, ORF8 evades the ER-associated degradation and accumulates in the ER lumen, induces ER stress, reshapes the ER to form intertwined and convoluted structures and accelerates the trafficking of secreted proteins. During B cell maturation, massive antibodies synthesis activates the unfolded protein response (UPR) to upregulate ER protein synthesis capacity. Interestingly, ORF8 contains an immunoglobulin-like domain, and likely acts as the "trojan horse" for SARS-CoV-2 to trick host cells to activate the UPR, thereby hijacking the ER function to facilitate virus assembly and maturation. The researchers also found that small molecule thiol reducing agents can reduce intermolecular disulfide bonds formed by ORF8 and accelerates its degradation, thereby alleviating ER stress.
The study reveals a new mechanism by which SARS-CoV-2 accessory protein ORF8 interferes with ER homeostasis in host cells. The discovery that ORF8 stability is redox-sensitive also provides new targets and interventions for viral infection.
Prof. WANG Lei and Associate Prof. WANG Xi from National Laboratory of Biomacromolecules, Institute of Biophysics, CAS are the co-corresponding authors of this paper, and Dr. LIU Ping is the first author. Prof. HU Junjie, Prof. ZHANG Hong and Prof. YANG Fuquan's group (Institute of Biophysics, CAS) make significant contributions to the work. The study was supported by the National Key R&D Program of China; the National Natural Science Foundation of China; the Strategic Priority Research Program of CAS and the Youth Innovation Promotion Association, CAS.
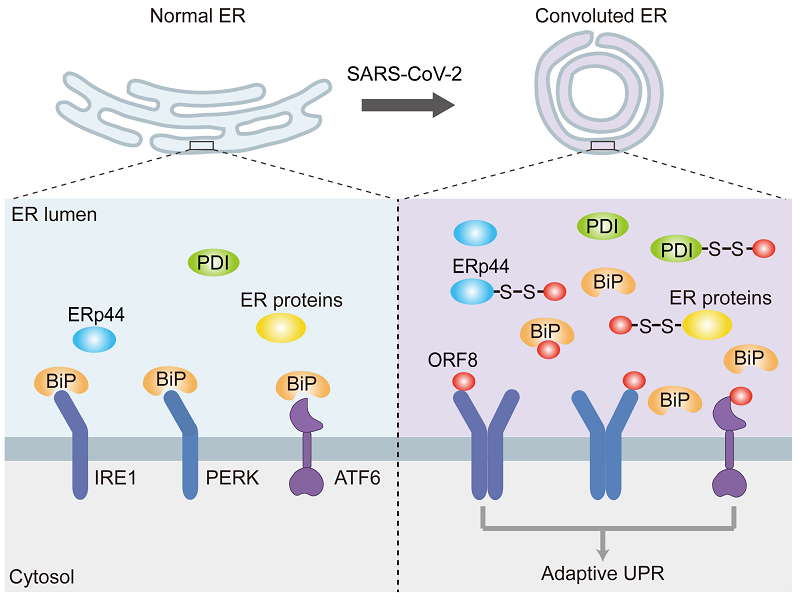
Proposed model for SARS-CoV-2 accessory protein ORF8-induced ER stress and ER remodeling.
Article link: https://www.sciencedirect.com/science/article/pii/S2213231722001604
Contact: WANG Lei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: wanglei@ibp.ac.cn
(Reported by Dr. WANG Chih-chen/Wang Lei's group)

