Researchers Reveal A Crucial Role of Ca2+ Transients on Cytosolic Surface of Endoplasmic Reticulum in Triggering Autophagosome Initiation Sites
Autophagy involves the engulfment of a portion of cytosolic contents in the autophagosome, and its subsequent delivery to the lysosome for degradation and recycling. Autophagy is crucial for cells to survive various stresses and also to maintain cellular homeostasis. The core steps of autophagosome formation involve the initiation of an isolation membrane (IM), and its expansion and closure. Autophagy in multicellular organisms exhibits fundamental differences from that in yeast. For example, yeast autophagosomes are generated at a single site on the vacuole. In mammalian cells, multiple IMs are initiated simultaneously on the ER, and IMs form extensive contacts with the ER during autophagosome biogenesis. A set of autophagy-related (Atg) genes identified from yeast genetic screens act at different steps of autophagosome formation. In yeast, upon autophagy induction, the Atg17/Atg13/Atg1 complex is targeted to the vacuolar membrane for IM initiation. In mammalian cells, the FIP200/ATG13/ULK1 complex (the Atg1 complex counterpart) and ATG9 vesicles are firstly targeted to the ER, which further recruit downstream ATG proteins for IM initiation. A long-standing unanswered question is the nature of the signal that triggers the assembly of the autophagosome-initiating Atg1 complex on the ER in mammalian cells and on the vacuole in yeast.
In a study published in Cell on October, 5, 2022, researchers from the Institute of Biophysics of Chinese Academy of Sciences demonstrated that Ca2+ transients on the ER surface trigger liquid-liquid phase separation of FIP200 to specify autophagosome initiation sites in multicellular organisms.
Prof. ZHANG Hong's group reported that autophagy stimuli trigger Ca2+ transients/oscillations on the outer surface of the ER membrane, whose amplitude, frequency and duration are controlled by the metazoan-specific ER transmembrane autophagy protein EPG-4/EI24. Persistent Ca2+ transients on the cytosolic ER surface in EI24-depleted cells cause accumulation of FIP200 autophagosome initiation complexes on the ER, and this autophagy defect is suppressed by attenuating Ca2+ oscillations. State-of-the-art multi-modal SIM analysis revealed that Ca2+ transients trigger the formation of highly dynamic and fusion-prone liquid-like FIP200 puncta. Multiple FIP200 puncta on the ER, whose association depends on the ER transmembrane proteins VAPA/B and ATL2/3, assemble into autophagosome formation sites. In addition, ATG9 vesicles modulate phase separation of FIP200 and spatial organization of FIP200 puncta on the ER. This study reveals a crucial role of Ca2+ transients on the cytosolic surface of the ER in triggering phase separation of FIP200 for specification of autophagosome initiation sites in multicellular organisms.
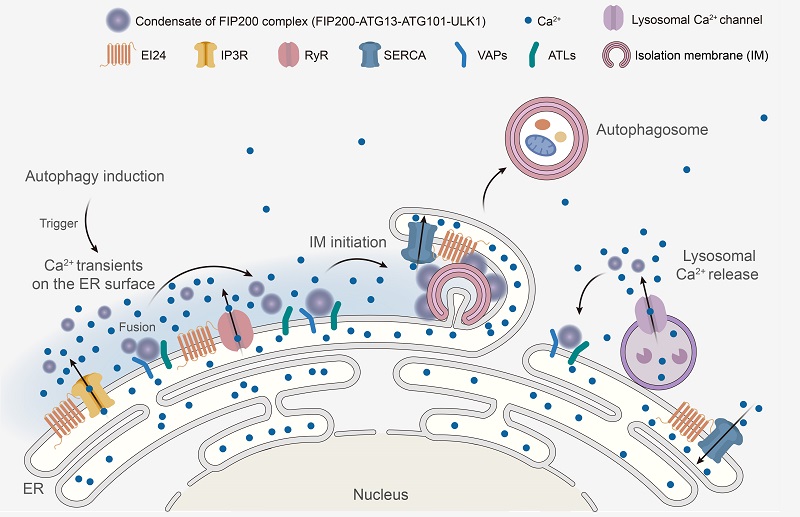
Figure. Model for the role of Ca2+ transients on the ER outer surface in the assembly of autophagosome initiation sites.
Article link: https://www.cell.com/cell/fulltext/S0092-8674(22)01123-0#%20
Contact: ZHANG Hong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: hognzhang@ibp.ac.cn
(Reported by Dr. ZHANG Hong's group)

