ATF4-dependent fructolysis fuels growth of glioblastoma multiforme
Glioblastoma multiforme (GBM) is a highly malignant brain tumor. GBM primarily utilizes glucose for energy production to support rapid proliferation of cancer cells under normal physiological conditions. GBM also utilizes other energetic substrates, such as fructose, amino acids, and fatty acids, for energy production. Epidemiological studies show that excessive fructose consumption is correlated with malignant tumor progression. In mammals, fructose metabolism pathway is different from glucose metabolism in the early stage. Fructose is transported by fructose transporter GLUT5 encoded by SLC2A5 gene. Intracellular fructose is phosphorylated by ketohexokinase to generate fructose 1-phosphate (F1P). Subsequently, F1P is split by aldolase B to produce glyceraldehyde (GA) and dihydroxyacetone phosphate (DHAP). GA and DHAP are then converted to glyceraldehyde 3-phosphate (GAP) by triokinase-mediated phosphorylation and isomerization, respectively, for merging with the later stage of glycolysis.
In mammals, Integrate Stress Response (ISR) includes the following four types: endoplasmic reticulum stress, amino acid deprivation stress, viral infection stress and heme deprivation stress, corresponding to activation of the following four protein kinases: PERK, GCN2, PKR and HRI. Previous studies have shown that these protein kinases can selectively activate the translation of the transcription factor ATF4 by phosphorylating the protein translation initiation factor eIF2α, thereby activating the expression of ATF4 target genes to complete the cellular stress response program.
Recently, a study published in Nature Communications by Dr. LI Xinjian's group reports that ATF4-dependent fructolysis fuels growth of glioblastoma multiforme. Mechanistically, glucose deprivation selectively activates ATF4 translation and ATF4 induces expression of two fructolytic genes SLC2A5 and ALDOB through binding to the promoter regions of these genes, as evidenced by ATF4 ChIP-Seq analysis. Disruption of ATF4-binding DNA sequences in the promoter regions of SLC2A5 and ALDOB by gene editing can effectively inhibit ISR-induced fructose metabolism. Functional studies demonstrate that genetically or pharmacologically blocking ISR-induced fructose metabolism significantly suppresses the fructose-mediated proliferation and clone formation of GBM cells under glucose-deprived condition. Moreover, results from mouse GBM xenograft study reveal that ISR resulted from glucose deprivation exists in GBM tissue and blocking fructose metabolism remarkedly retards GBM growth. Collectively, these data suggest that fructose is an alternative energy-supplying nutrient for glucose-deprived GBM, thus there is a caveat which concerns about the GBM patients with a high-fructose diet and developing small-molecule compounds targeting fructose metabolism may be utilized for treating human GBM.
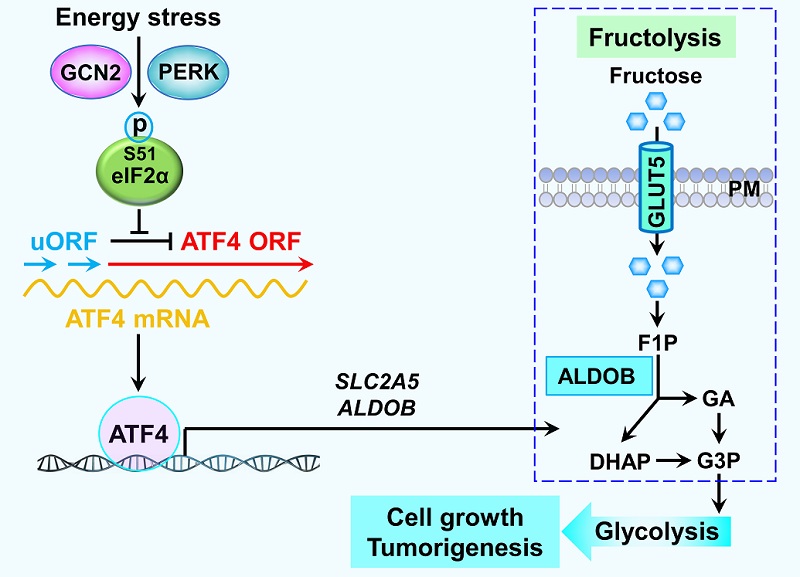
Figure. A mechanism of ATF4-dependent fructolysis supports GBM growth under glucose-deprived condition. A model shows that ATF4, which is activated upon glucose deprivation, induces expression of GLUT5 and ALDOB. High levels of GLUT5 and ALDOB drive fructolysis to maintain GBM growth under glucose-deprived condition.
Article link: https://www.nature.com/articles/s41467-022-33859-9
Contact: LI Xinjian
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: lixinjian@ibp.ac.cn
(Reported by Dr. LI Xinjian's group)

