Researchers Reveal the Formation of Herpesvirus Assembly Compartments Mediated by Liquid-liquid Phase Separation
Summary:
A mature herpesvirion contains dozens of structural proteins, whose assembly in the vast cytosolic space is a daunting task. Prof. DENG Hongyu's group from the Institute of Biophysics of the Chinese Academy of Sciences, uncovered liquid-liquid phase separation as a strategy to form cytoplasmic virion assembly compartments, mediated by a viral tegument protein, to facilitate virus assembly.
Main Text:
As intracellular parasites, viruses must modulate the environment of infected cells to facilitate processes which are crucial for their own propagation, including virion assembly. This is an essential yet complex step in the virus life cycle, particularly so for viruses with large genome sizes (i.e. large coding capacities for many structural proteins) and complicated virion structures such as herpesviruses.
Herpesviridae is a large family of DNA viruses that infect humans and other animals. A mature herpesvirion contains, from the inside to the outside, double-stranded DNA genome, capsid, tegument and envelope. Assembly of the dozens of structural proteins into mature virions in the vast cytosolic space is a daunting task, and the underlying mechanisms remained poorly understood.
In a study published in Journal of Cell Biology, a research group led by Prof. DENG Hongyu from the Institute of Biophysics of the Chinese Academy of Sciences, demonstrated the formation of cytoplasmic virion assembly compartments (cVACs), which recruit viral tegument proteins and host vesicles containing viral glycoproteins for virus maturation, during γ-herpesvirus infection. They showed that cVACs are membraneless compartments generated through a process of liquid-liquid phase separation (LLPS), mediated by a tegument protein, revealing the critical role of phase separation in γ-herpesvirus assembly.
Prof. Deng's group has been investigating the mechanisms involved in virion assembly and has previously identified a number of tegument proteins which play essential roles in virion morphogenesis. To investigate cytoplasmic virion assembly of γ-herpesviruses, the authors first constructed recombinant murine gammaherpesvirus 68 (MHV-68) expressing a fluorescently-tagged tegument protein. Using live-cell imaging and correlative light and electron microscopy, they discovered the formation of cVACs which start to accumulate in the cytoplasm as viral infection proceeds. They further showed that cVACs are membrane-less structures and are formed by LLPS.
By examining a series of viral tegument proteins, the researchers identified ORF52, an abundant tegument protein in virion, that possesses LLPS properties. Analysis of an ORF52-null viral mutant indicated that ORF52 is required for cVAC formation as well as complete tegumentation and secondary envelopment of viral particles.
Furthermore, the researchers found that both DNA and RNA can drive LLPS of ORF52 in vitro. However, during the late stage of viral replication, RNA colocalizes with ORF52 puncta and is likely to promote LLPS of ORF52.
Finally, by deleting domains or mutating a conserved cluster of basic amino acids in the intrinsically disordered region of ORF52 followed by ultrastructural and functional analyses, the researchers demonstrated that the LLPS properties of ORF52 are crucial for cVACs formation and virion production.
In summary, this study defines herpesvirus cVACs as membraneless compartments that are generated through LLPS, mediated by an abundant tegument protein. The study adds to the cellular processes that are facilitated by phase separation. It also provides novel insights into cVACs biogenesis and lays a foundation for further investigation of the complete details of herpesvirion morphogenesis.
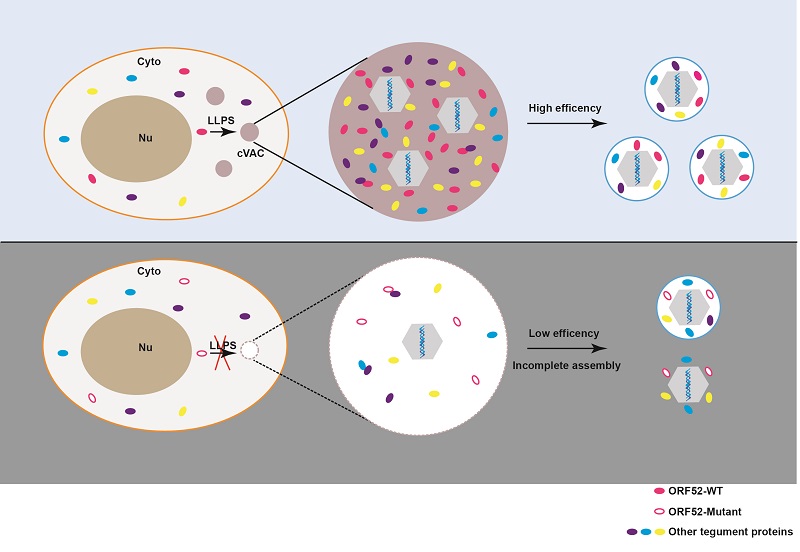
A Model depicting cVAC formation and virus assembly mediated by phase separation of ORF52
(Image by Zhou Sheng)
Article link: https://doi.org/10.1083/jcb.202201088
Contact: DENG Hongyu
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: hydeng@ibp.ac.cn
(Reported by Dr. DENG Hongyu's group)

