Engineered anti-PDL1 with IFNα targets both immunoinhibitory and activating signals in the liver to break HBV immune tolerance
Although effective prophylactic hepatitis B virus (HBV) vaccines have been available since the 1990s, an estimated 257 million people worldwide are living with chronic HBV infection, which poses a high risk for developing fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). Type I interferons (IFNs), one type of FDA-approved drug, cannot effectively reduce the viral antigen load and hardly maintain sustained viral control after treatment withdrawal. Long-term repeated high-dose IFN treatment can cause systemic toxicity. In addition, IFNs can upregulate the expression of programmed death ligand 1 (PDL1), which in turn suppresses the antiviral immune response and gradually reduces the therapeutic effect. How to overcome the side effects and immunosuppression caused by systemic administration of IFN and achieve a functional cure for CHB is a challenging problem.
Recently, a study published in the Gut by Prof. PENG Hua at the Institute of Biophysics of the Chinese Academy of Sciences, and Prof. FU Yang-Xin at the School of Medicine, Tsinghua University reports an anti-PDL1-IFNα heterodimeric fusion protein for CHB treatment. One arm of anti-PDL1-IFNα was derived from the anti-PDL1 antibody, and the other arm was IFNα, allowing targeted delivery of IFNα into the liver by the anti-PDL1 antibody. The researchers found that anti-PDL1-IFNα multi-functional fusion protein could block immune checkpoint PDL1, target IFNα to the liver for suppressing HBV replication, and promote DC maturation and antigen presentation, providing a "window" for breaking HBV-induced immune tolerance. A subsequent combination with HBsAg vaccination induced a strong anti-HBsAg immune response for CHB functional cure.
The study reveals several mechanisms that provide critical information for future CHB therapy. First, anti-PDL1-IFNα therapy appears to be a short-term treatment effective for targeting IFNα to the PDL1+ intrahepatic cells and less toxic than the prolonged high-dose treatment with IFNs. Second, anti-PDL1-IFNα could deliver immunomodulatory molecules that reduce PDL1-mediated immune tolerance through both checkpoint blockades and enhancement of DCs' function. Third, anti-PDL1-IFNα created a positive synergistic stimulating response between checkpoint blockades and IFNα signaling and might further enhance the targeting effect through IFNα-induced PDL1 upregulation.
Collectively, anti-PDL1-IFNα therapy combined with HBsAg vaccine administration could induce a high-level, persistent anti-HBsAg response for efficient viral clearance and protective immunity in a chronic HBV mouse model. This study offers a promising translatable combination therapeutic strategy for achieving the CHB functional cure of CHB.
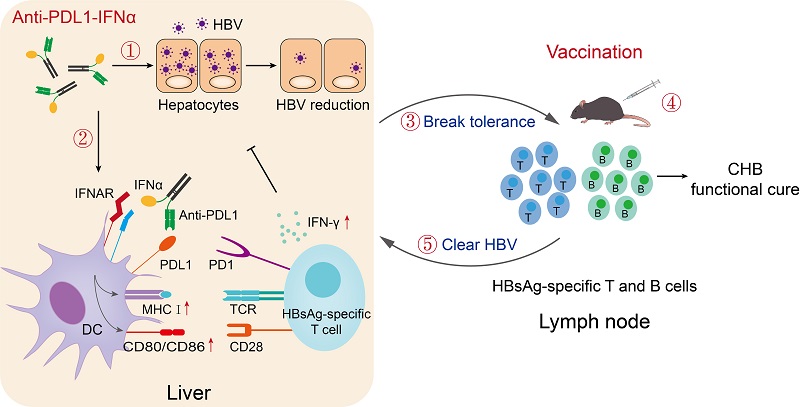
Figure. Anti-PDL1-IFNα fusion protein combined with the HBV vaccine to achieve the functional cure of CHB
Article link:https://gut.bmj.com/content/early/2022/10/31/gutjnl-2022-327059
Reference: Chao-Yang Meng, Shiyu Sun, Yong Liang, Hairong Xu, Chao Zhang, Min Zhang, Fu-Sheng Wang, Yang-Xin Fu, Hua Peng. Engineered anti-PDL1 with IFNα targets both immunoinhibitory and activating signals in the liver to break HBV immune tolerance. Gut. October 31, 2022. DOI: 10.1136/gutjnl-2022-327059
Contact: PENG Hua
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Tel: 86-010-64881152
E-mail: hpeng@ibp.ac.cn
(Reported by Dr. PENG Hua's group)

