Tumor cells employ the unfolded protein response to drive immunosuppression non-cell-autonomously
A feature in the tumor microenvironment is endoplasmic reticulum (ER) stress, which refers to a condition when unfolded proteins accumulate in the ER lumen. Intrinsic and extrinsic perturbations, such as gene mutations, protein overload, and hypoxia, may elicit ER stress and induce the downstream unfolded protein response (UPR). Previous studies have revealed the importance of the UPR in tumor growth and metastasis, as well as its physiological functions in immune cell regulation. In a recent publication in Cell Metabolism, 'Cancer cell-intrinsic XBP1 drives immunosuppressive reprogramming of intratumoral myeloid cells by promoting cholesterol production' (https://doi.org/10.1016/j.cmet.2022.10.010), Dr. Zaili Yang et. al. from the laboratory of Dr. Likun Wang in the Institute of Biophysics, Chinese Academy of Sciences reported that tumor cells experiencing ER stress non-cell-autonomously suppressed anti-tumor immunity to facilitate tumor growth by secreting cholesterol via small extracellular vesicles (sEVs) to foster fostering the expansion and activation of myeloid-derived suppressor cells (MDSCs).
By employing B16 mouse melanoma cells or MC38 mouse colon adenocarcinoma cells that have XBP1 (a transcription factor that is downstream of the IRE1α pathway, the most conserved UPR branch) knocked down (shXBP1), Yang et. al. found that shXBP1 greatly retarded the tumor growth in immunocompetent, but not immunodeficient, mice, suggesting that tumor-intrinsic XBP1 may regulate immune response. Further analysis using mass cytometry and flow cytometry revealed a decreased proportion of MDSCs in tumor-infiltrating leukocytes (TILs), accompanied by increased level of CD8+ T cells, in shXBP1 tumors compared to its wild-type (shNT) counterparts. Depletion of MDSCs in mice bearing shNT tumors also elevated the ratio of CD8+ T cells and suppressed tumor growth.
Transcriptome analysis on tumor tissues identified genes encoding enzymes of cholesterol biosynthesis whose transcription levels were downregulated in tumors upon XBP1 depletion. Meanwhile, a decreased level of cholesterol in tumor tissues was also observed. In vitro and in vivo experiments showed that cholesterol can stimulate the expansion and activation of MDSCs. On the other hand, genetic or pharmacological disruption of cholesterol production in tumor cells decreased the proportion of MDSCs and increased CD8+ T cells in TILs, and reduced tumor growth rate. Remarkably, XBP1s (the spliced and active form of XBP1) binds to the promoter of HMGCR, a rate-limiting enzyme-encoding gene for cholesterol biosynthesis, and directly promotes the transcription of HMGCR. Thus, the UPR protein XBP1s drives cholesterol production in tumor cells to suppress anti-tumor immunity and facilitate tumor growth.
The authors then investigated the mechanism how cholesterol propagated ER stress signals from tumor cells to MDSCs. They found that shXBP1 reduced the amount of small extracellular vesicles (sEVs) secreted by tumor cells, and decreased the cholesterol content in sEVs. Meanwhile, sEVs derived from shXBP1 tumor cells were less effective in promoting MDSCs expansion and activation compared with the ones from shNT tumor cells. Furthermore, inhibition of macropinocytosis on MDSCs limited the sEVs-induced proliferation and activation of MDSCs. These results indicate that cholesterol is delivered from tumor cells via sEVs, which are then received by MDSCs through macropinocytosis.
Finally, the authors proposed a strategy in tumor therapy, in which inhibiting IRE1α/XBP1 pathway by using KIRA8 greatly revived anti-tumor immunity and reduced tumor growth. Such inhibitory effect can be further improved when combined with anti-PD-1 checkpoint blockade.
In summary, this work reveals an interesting function of tumor-intrinsic UPR, which dictates the production of cholesterol and leads to immunosuppression by orchestrating MDSCs in a cell non-autonomous way, and provides new insight into tumor therapy.
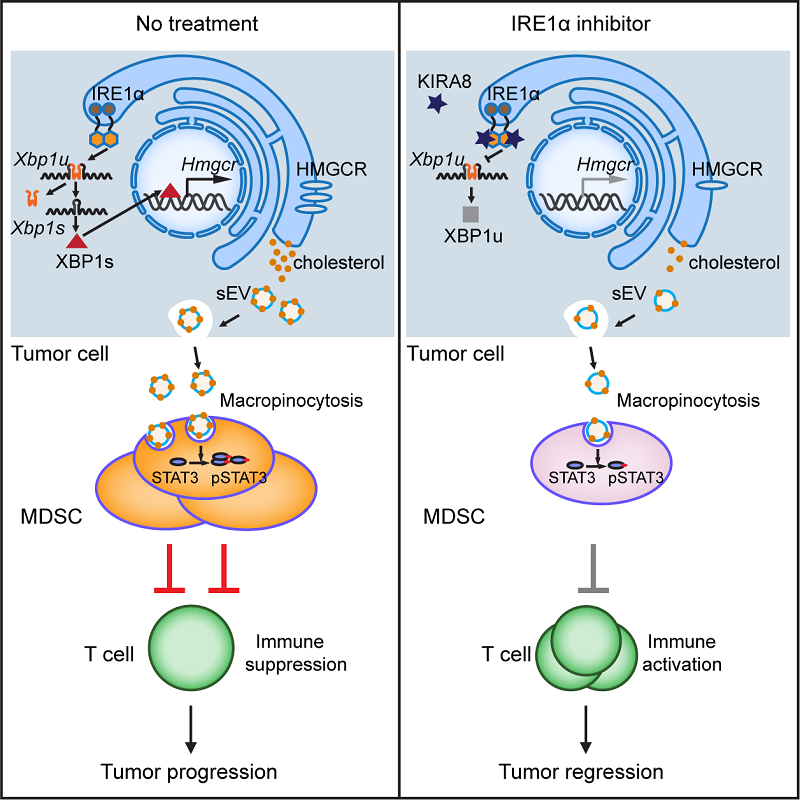
Figure. Proposed model showing how XBP1 in tumor cells drives immunosuppressive reprogramming and IRE1α inhibitor reactivates anti-tumor immune response, thus limiting tumor growth (cited from https://doi.org/10.1016/j.cmet.2022.10.010).
Dr. YANG Zaili is the first author, and Dr. WANG Likun is the corresponding author. For more information, please refer to the original article (https://doi.org/10.1016/j.cmet.2022.10.010).
Article link: https://www.sciencedirect.com/science/article/pii/S1550413122004612?via%3Dihub
Contact: WANG Likun
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: wanglikun@ibp.ac.cn
(Reported by Dr. WANG Likun's group)

