The molecular mechanisms for the different cysteine reactivity of two major human cytosolic Hsp70 homologues
Hsp70s are highly conserved molecular chaperones that are involved in many crucial cellular roles, in particular maintaining protein homeostasis. Hsp70s are also involved in disease mechanisms and established as drug targets, including for cancer as well as neurodegenerative and infectious diseases. Human HspA1A (hHsp70) and HspA8 (hHsc70) are the major cytosolic Hsp70s and they have overlapped and nonoverlapped functions. Post-translational modifications (PTMs) of chaperones are the chaperone code to finely regulate chaperone activity and to integrate molecular chaperones into the cellular signaling network. Cysteine is unique in terms of its nucleophilicity, and can undergo oxidative modifications upon redox and covalent modifications with electrophiles including certain drugs. hHsp70 contains five cysteine residues (C17, C267, C306, C574 and C306) and hHsc70 contains four (C17, C267, C574 and C306). Previous studies have shown these Cys residues can undergo different cysteine modifications to regulate their functions, and hHsp70 and hHsc70 have different cysteine reactivity.
In this study the mechanisms behind the different cysteine reactivity of hHsp70 and hHsc70 were addressing by studying the factors which determine the cysteine reactivity by Ellman assay for the quantification of accessible free thiols and NMR analysis for the assessment of structural dynamics. The results showed that the lower cysteine reactivity of hHsc70 is probably due to the lower structural dynamics of hHsc70 and the stronger inhibition effect of interaction between the SBDα and SBDβ on the cysteine reactivity of the SBDα in hHsc70. The flexibility of Gly557 in the loop between αB and αC (LBC) of hHsp70 SBDα contributes significantly to the higher structural dynamics and cysteine reactivity of the hHsp70 SBDα.
The different cysteine reactivity between hHsp70 and hHsc70 may contribute to the differences in function and functional regulation between the two proteins, which could provide strategies for designing specific Hsp70 inhibitors. The results suggest that the cysteine reactivity of Hsp70 is determined by structural dynamics, allosteric conformational changes and domain communication, and the effect of these factors may be specific to different Hsp70 homologues and different cysteine modifications. The overall cysteine reactivity of hHsp70 and hHsc70 is an important reference point to predict the redox-regulated chaperone activity in vivo and to design new covalent inhibitors, and our results are also fundamental to the study of crosstalk between cysteine modifications and other PTMs in Hsp70s. The study also highlights that structural dynamics of a protein could significantly affect its cysteine reactivity, its sensitivity to redox and the potential regulation by redox.
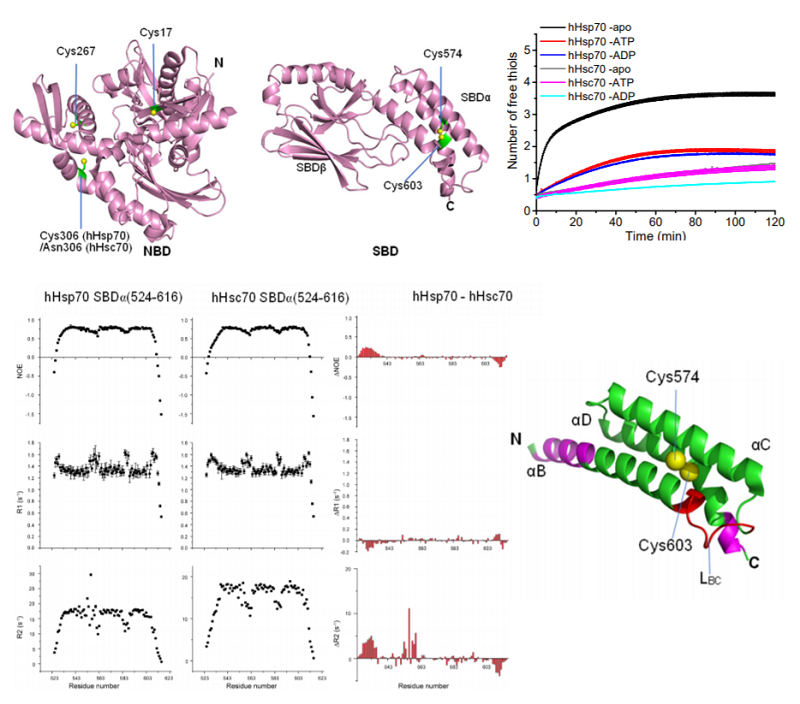
The distribution of cysteine residues in the structure of human HspA1A (hHsp70) and HspA8 (hHsc70), the different cysteine reactivity of hHsp70 and hHsc70 measured by DTNB assays and the different structural dynamics of hHsp70 SBDα and hHsc70 SBDα measured by NMR.
This study was carried out by the group of Prof. Sarah Perrett in the Institute of Biophysics, Chinese Academy of Sciences, and was published online in the Journal of Biological Chemistry on 22 Nov 2022.
Article link: https://www.sciencedirect.com/science/article/pii/S0021925822011668?via%3Dihub
Contact: ZHANG Hong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: zhangh@ibp.ac.cn
(Reported by Dr. Sarah Perrett's group)

