Researchers Develop Novel Correlated Light, Ion and Electron microscopy (CLIEM) for site-specific preparation of cell lamellae for cryo-electron tomography
Cryo-electron tomography (cryo-ET) is a leading technique for the structure determination of cells in situ. One limitation of cryo-ET is its restricted imaging depth of typically several hundred nanometers. Therefore, thinning the cells to lamella is required before applying cryo-ET. Cryogenic focused ion beam (cryo-FIB) milling is a promising method for preparing lamella of vitrified biological samples. However, conventional FIB milling does not allow the fabrication of lamella at specific sites due to the inability to locate the target inside the cell.
On January 16th, 2023, an article led by Prof. XU Tao, Prof. JI Wei and Prof. GUO Qiang, entitled "Integrated multimodality microscope for accurate and efficient target-guided cryo-lamellae preparation" was published in Nature Methods.
In this study, Scientists developed a novel cryogenic Correlated Light, Ion and Electron microscopy (cryo-CLIEM) system to solve the problem mentioned above. CLIEM integrated a confocal microscope into a commercial dual-beam scanning electron microscope, and provided new possibilities to prepare cell lamella containing specific targets with high accuracy and efficiency for cryo-ET. Based on CLIEM, they also developed an efficient working routine to register the light and FIB images, without the need to use fiducial markers and coordinate transformation. They etched a pattern using FIB as registration benchmark, and projected the 3D light microscopy (LM) image to the FIB angle. The projected image, termed "LM vie FIB", can be directly correlated with the FIB image.
Using CLIEM, they investigated cell organelle interactions between lipid droplet and mitochondria, as well as between mitochondria and endoplasmic reticulum in HepG2 cells. They prepared cell lamellae precisely containing these structures, and discovered abundant tethers at the contact sites in situ using cryo-ET. Furthermore, they tackled the native structure of centrosome in HeLa cells, which is a very rare event in mammalian cells. They succeeded in preparing lamella containing single centriole using CLIEM, and resolved the in situ structure of a centriole and the surrounding cellular structures using cryo-ET.
Surprisingly, in the native centriole, they discovered a ring structure with a diameter of approximately 100 nm, which contained 27 evenly distributed rod-like electron densities. The function of this structure still remains unclear. The above results demonstrated that CLIEM is a very efficient and accuracy method for site-specific preparation of cell lamella, and can be applied to a variety of biological systems for the in situ structure investigation using cryo-ET.
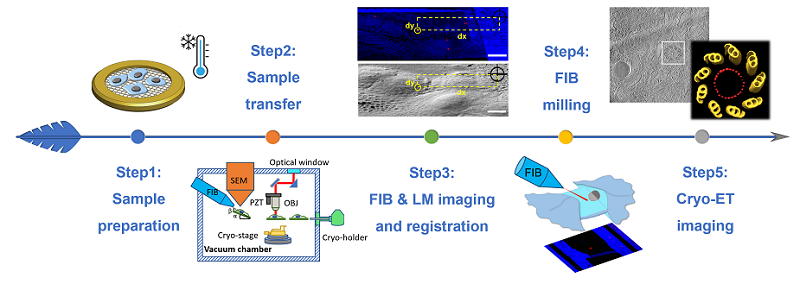
Workflow of CLIEM
This study was funded by the National Key Research and Development Program of China, the National Natural Science Foundation of China, the Strategic Priority Research Program and the Youth Innovation Promotion Association of CAS, etc.
Article link:
https://www.nature.com/articles/s41592-022-01749-z
Related Article:
ELI TriScope:https://www.nature.com/articles/s41592-022-01748-0
Contact: JI Wei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: jiwei@ibp.ac.cn
(Reported by Dr. JI Wei's group)

