Institute of Biophysics invented novel instruments to achieve highly effecient and precise cryo correlative fluorescence and electron microscopy for in situ structural study of cells
Nanomachines and ultrastructures inside cells are the basic units involved in life activities. They perform specific physiological functions through close cooperation with each other. Seeing is believing. Studying the in situ assembly and function of these complicated and precise nanostructures has been a hot topic in life science. Cryo-electron tomography (cryo-ET) is currently the main technique for in situ structural analysis. However, due to the limitation of electron beam penetration, cell and tissue samples should be milled to lamella of ~200 nanometers using focused ion beams (FIB) for imaging. However, the random milling technique brings great challenges to the study of specific targets with relatively low abundance in cells. The prepared cryo-lamella sample often fails to retain the target of interest.
To deal with this technical bottleneck, teams from Institute of Biophysics, Chinese Academy of Sciences developed two different types of precise systems to prepare target cryo-lamellae. On January 16, they published back-to-back papers in Nature Methods, entitled "ELI trifocal microscope: a precise system to prepare target cryo-lamellae for in situ cryo-ET study" and "Integrated multimodality microscope for accurate and efficient target-guided cryo-lamellae preparation". They proposed a scheme to use fluorescence imaging to navigate cryo-FIB milling, and separately developed the integrated correlative light and electron microscopy (CLEM) cryo-FIB system, named as ELI-TriScope and CLIEM respectively (Fig. 1).
The ELI-TriScope system uses a commercial dual-beam scanning electron microscope modified to incorporate a cryo-holder-based transfer system and embed an optical imaging system (cryogenic SimulTAneous monitoR system, cryo-STAR) just underneath the vitrified specimen. It sets electron, light, and ion beams at the same focal point to achieve accurate and efficient preparation of a target cryo-lamella. Cryo-FIB milling can be accurately navigated by monitoring the real-time fluorescence signal of the target molecule.
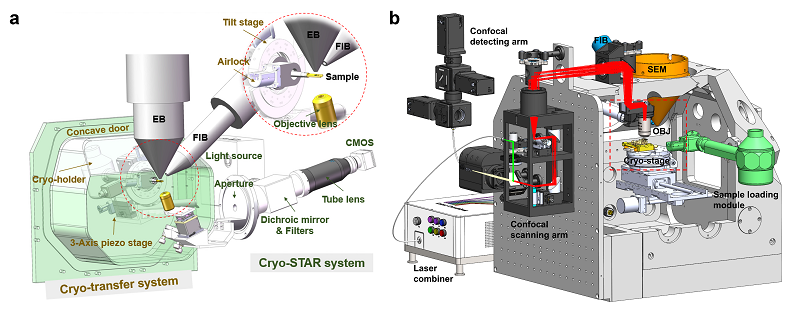
Figure 1. Design of ELI-TriScope (a) and CLIEM (b).
The CLIEM system incorporates a 3D multi-color confocal microscope into a dual-beam FIB-SEM system. Through high-quality whole-cell multicolor three-dimensional (3D) fluorescence imaging, the 3D spatial position of the research target can be accurately located. Through ingenious projection transformation, the light and FIB images can be rapidly and accurately correlated, achieving the FIB milling with a high precision of tens of nanometers. In addition, the team developed a "virtual lamella" function that generates virtual lamella of specific positions in the cell, which helps to determine the optimal milling site in a crowded cell.
Using the above systems, the research team efficiently prepared cryo-lamellae of cells. Through subsequent cryo-electron tomography (cryo-ET) analysis, they discovered new in situ structural features of human centrosomes, and researched cellular ultrastructure such as organelle interaction sites (Figure 2). These results demonstrate that ELI-TriScope and CLIEM, as advanced, precise, and efficient cryo-FIB techniques, provide new solutions for site-specific sample preparation of cryo-ET. They have a wide range of potential applications in the study of the ultrastructure of specific events in cells and are expected to advance the development of in situ structural biology in the future.
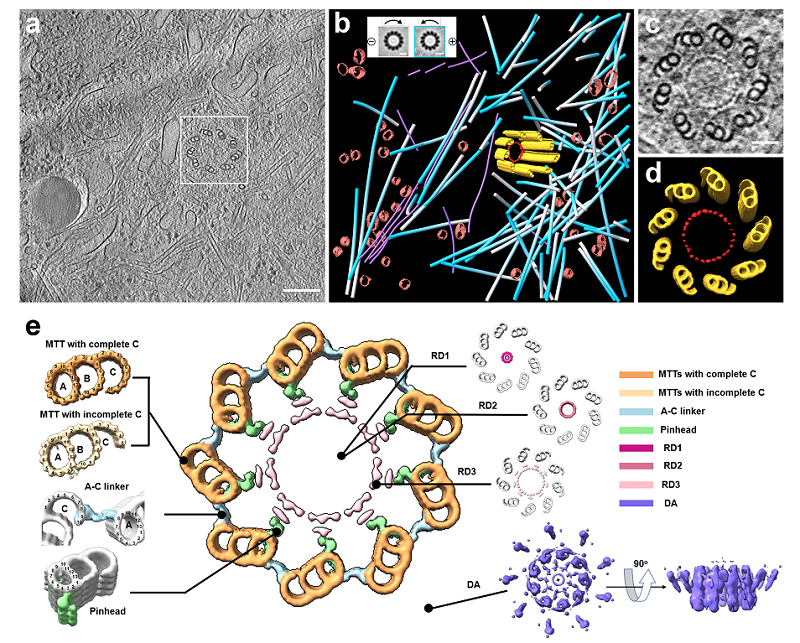
Figure 2. (a) Transmission electron microscopy (TEM) of cryo-lamella containing centrosome. (b-e) In situ structure of human centrioles. The central large image shows a cross-section of human centrioles resolved by cryo-ET. MTTs, microtubule triplets; RD1-3, the ring-like inner density of centrioles; DA, distal appendages. A-C linker, A- and C-tubule connection region. Pinhead, pinhead-like structure.
SUN Fei (Professor), JI Gang (Professor of Engineering), and ZHU Yun (Professor), from Institute of Biophysics, Chinese Academy of Sciences, are co-corresponding authors of ELI-TriScope work. LI Shuoguo (Senior Engineering) from Center for Biological Imaging, Core Facilities for Protein Science, and WANG Ziyan from SUN Fei's lab, are co-first authors of this work.
XU Tao (Academician) and JI Wei (Professor) from Institute of Biophysics, Chinese Academy of Sciences, and GUO Qiang (Professor) from Peking University, are co-corresponding authors of CLIEM work. LI Weixing (Senior Engineering), LU Jing (Senior Engineering), and XIAO Ke (Engineering), from Institute of Biophysics, Chinese Academy of Sciences, and ZHOU Maoge (Associate Professor) from Guangzhou Laboratory, are co-first authors of this work.
These researches were supported by grants from the National Key Research and Development Program, the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, Main Direction Program of Knowledge Innovation of Chinese Academy of Sciences. Sample preparation, data collection and analysis are supported by the staff of Center for Biological Imaging, Core Facilities for Protein Science, Institute of Biophysics.
Links to papers:
ELI TriScope:https://www.nature.com/articles/s41592-022-01748-0
CLIEM:https://www.nature.com/articles/s41592-022-01749-z
Links to related news:
http://english.ibp.cas.cn/research_23463/Research_progress/202301/t20230113_326307.html
http://english.ibp.cas.cn/research_23463/Research_progress/202301/t20230113_326295.html
Contact: SUN Fei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: feisun@ibp.ac.cn
(Reported by Dr. SUN Fei's group)

