Researchers develop a small molecule fusion agonist that repairs mitochondrial damages
In a study published in Nature Chemical Biology, researchers from the Chinese Academy of Sciences and Nankai University collaboratively report a small molecule compound named S89 that can specifically activate mitochondrial outer membrane fusion protein MFN1 and repair multiple types of mitochondrial damage.
Mitochondria, as the center of regulation of cellular energy and metabolism homeostasis, maintain normal functions through continuous fusion and fission. Excessive fragmentation of mitochondria is one of the important signs of many human diseases and aging. The molecular machines involved in mitochondrial dynamics are members of the dynamin superfamily, and the mitochondrial outer membrane fusion is mediated by mitofusins (MFN1 and MFN2). Mutations in MFN2 lead to a variety of inherited neurodegenerative diseases such as Charcot-Marie-Tooth disease (CMT2A). Thus, the development of compounds that can promote mitochondrial fusion would contribute to the treatment of mitochondria-related diseases.
The research groups found through the screening that S89, a derivative of the natural product extracted from spirea, has a robust effect on promoting mitochondrial fusion. The in vitro studies showed that S89 binds directly to a loose region of HB2 of purified MFN1. The binding of a loose region of HB2 to GTPases locks the molecule in an autoinhibited manner, which can be lifted by the competitive binding of S89. Likewise, site-specific mutations in HB2 or the binding of GTP can disentangle the MFN1 autoinhibition. It is worth noting that S89 has no effect on the MFN1 homologous protein MFN2 due to a stronger autoinhibition.
The researchers verified the biological effects of S89 using a variety of cell models, i.e. cells from patients with MELAS syndrome, cells from patients with CMT2A, cells treated with oxidative stress inducer paraquat, and ferroptosis inducer RLS3. In addition, this study also showed that S89 can alleviate the myocardial damage caused by ischemia and reperfusion injury in mice.
This study unraveled the mystery of mitochondrial outer membrane fusion. The reversible and controllable efficacy of S89 provides great application prospects by further activating endogenous healthy MFN1 for the treatment of mitochondrial fragmentation-related diseases.
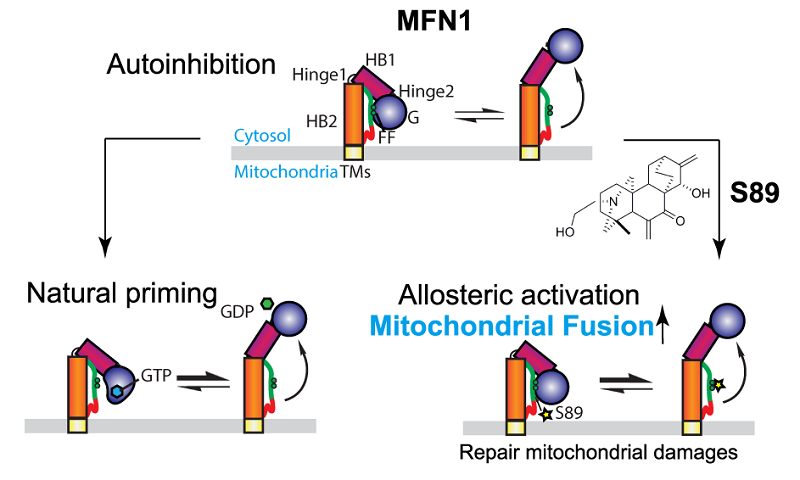
Schematic model for the conserved phenylananine (FF), nucleotide (GTP), and S89-regulated locking or unlocking mechanism of mitochondrial fusion protein MFN1
This work was supported by the National Natural Science Foundation of China, the National Key R&D Program, the Strategic Priority Research Program (Category B), and the Project for Young Scientists in Basic Research of the Chinese Academy of Sciences.
Contact: HU Junjie
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: huj@ibp.ac.cn
(Reported by Dr. HU Junjie's group)

