Collaboration between the ZHANG Xinzheng and XIANG Ye groups reveals a novel receptor binding mode and mechanism of cross-species transmission in alphavirus
On April 24, 2023, the article titled "Structure of Semliki Forest virus in complex with its receptor VLDLR" by the research groups of ZHANG Xinzheng at the Institute of Biophysics, Chinese Academy of Sciences (CAS) and XIANG Ye at the School of Medicine, Tsinghua University was published online in the journal Cell. In this study, high-resolution cryo-electron microscopy was used to resolve the structure of Semliki Forest virus (SFV) in complex with its human receptor very-low-density lipoprotein receptor (VLDLR), identify a novel receptor binding site for alphavirus, and reveal a novel mechanism by which multiple similar tandem domains of VLDLR bind synergistically and specifically to SFV. This study is the first to elucidate the molecular mechanism of cross-species transmission of viruses mediated by a universal receptor molecule, which provides novel targets for the design and development of antiviral drugs and vaccines.
Alphaviruses are enveloped, single-stranded, positive-sense RNA viruses in the Togaviridae family that are transmitted by mosquitoes and other arthropods and infect a variety of hosts, including humans, birds, rodents, and horses. In humans, alphaviruses can cause fever, severe and persistent headaches, muscle pain, and joint pain. Some alphaviruses can infect neurons and cause central nervous system infections, leading to severe encephalitis. To date, no drugs or vaccines have been approved to treat or prevent alphavirus infections. In recent years, there have been several major advances in the study of the mechanisms of alphavirus infections. The molecular mechanism of binding of chikungunya virus (CHIKV) to its receptor Mxra8 (matrix remodeling associated protein 8) and Venezuelan equine encephalitis virus (VEEV) to its receptor LDLRAD3 (low-density lipoprotein receptor class A domain-containing protein 3) have been elucidated. However, current studies do not yet explain why a wide range of species, from invertebrates to vertebrates and across a wide range of phylogenetic relationships, can be infected by alphavirus. In 2021, VLDLR was found to be a universal receptor that facilitates infection of multiple species, including mosquitoes, mice, horses, and humans, by many alphaviruses, including SFV. Very few universal receptors that mediate viral transmission across vastly different hosts have been reported. Moreover, the mode of binding of these viruses to such receptors and the mechanism by which cross-species transmission occurs are unknown.
There are 80 trimeric forms of the spike protein on the surface of the alphavirus envelope, with each spike consisting of three E1/E2 heterodimers. E1, located in the membrane-proximal region of the spike, is generally considered to be primarily responsible for virus-host membrane fusion, while E2, located in the membrane-distal region of the spike, participates in receptor binding. Structural studies of the CHIKV/MXRA8 and VEEV/LDLRAD3 complexes showed that LDLRAD3 and MXRA8 both bind to the cleft formed by two adjacent E1-E2 heterodimers in one viral spike, and both E1 and E2 participate in receptor binding. In this study, the researchers successfully resolved the high-resolution structure of SFV VLP in complex with the receptor VLDLR using block-based reconstruction algorithm and unexpectedly found that VLDLR binds to the DIII domain of the membrane-proximal region of SFV E1 (E1-DIII) and that SFV E2 is not involved in binding to VLDLR. This binding mode completely differs from previously known alphavirus receptor binding modes, thereby updating our understanding of the interaction between alphaviruses and their receptors.
The research groups further found that VLDLR binds to SFV E1-DIII through multiple extracellular cysteine-rich repeat sequence domains (LDLR class A repeats, LAs). A single LA domain binds weakly to the virus, with a dissociation constant of 1 ?M-30 ?M, whereas multiple tandem LA domains bind significantly more strongly to the virus, with a dissociation constant of 1 nM-100 nM. The SFV surface receptor binding sites E1-DIII are densely packed together in the 2-fold and 5-fold axes, possibly providing the necessary conditions for synergistic binding of multiple LA structural domains. By analyzing the structure of SFV VLP and multiple tandem LA complexes, the researchers found that multiple tandem LAs could simultaneously bind multiple E1-DIII sites in SFV VLP in multiple different binding modes instead of through a fixed pattern (Figure 1). Near the 5-fold axis, multiple tandem LAs undergo a slight rotation to avoid steric effects and synergistically bind E1-DIII. Compared to the binding of a single LA, some interactions with tandem LAs at critical sites remain even though most of the interactions with E1-DIII are disrupted during rotation. In addition, the loss of non-critical interactions with a single LA is compensated by the synergistic effect of the tandem LAs, which greatly enhances viral binding to its receptor. This mode of synergistic interaction through multiple tandem LAs increases the ability of the receptor to tolerate the loss of binding capacity in individual LAs. Although highly divergent, the key residues associated with viral interaction, W132, E137, and D139, are fully conserved in most LAs in VLDLR from different species. The unique synergistic binding mode of VLDLR allows it to tolerate sequence differences between species from different clades when mediating viral infection, enabling viral transmission across diverse species. In summary, this study uses structural biology and various biochemical approaches to illustrate for the first time the molecular mechanism by which relatively conserved universal receptors in multiple hosts synergistically and specifically bind with viruses through weak interactions with multiple similar structural domains to mediate cross-species viral transmission.
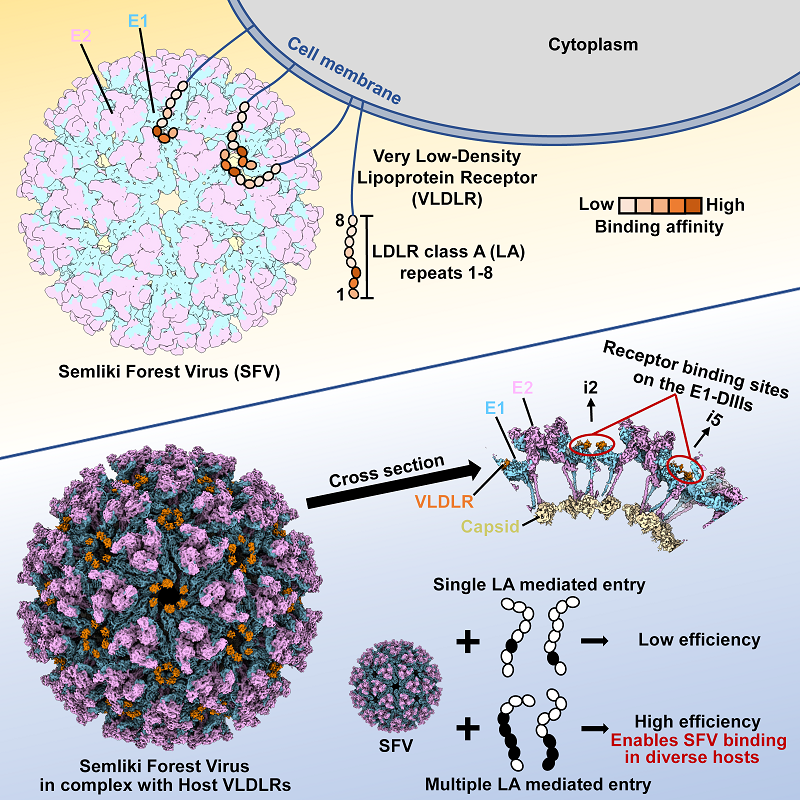
Figure 1. Novel receptors mediate cross-species transmission of the alphavirus SFV via synergism between multiple consecutive LA repeats
(Source: Cao et al., 2023, Cell 186, 1-11)
XIANG Ye of the School of Medicine, Tsinghua University, and ZHANG Xinzheng of the Institute of Biophysics, Chinese Academy of Sciences are the co-corresponding authors of this article. CAO Duanfang and CAO Ziyi from the Zhang lab and MA Bingting from the Xiang lab are the paper's co-first authors. This work was supported by funds from the Key Research and Development Program of the Ministry of Science and Technology of China, the Tsinghua University Vanke Special Fund for Public Health and Health Discipline Development, the National Natural Science Foundation of China, the Tsinghua University Spring Breeze Fund, the Beijing Center for Frontier Research Center for Biological Structures, the Strategic Pioneer Science and Technology Special Project of the Chinese Academy of Sciences, the Basic Research Frontier Science Program of the Chinese Academy of Sciences, and the Youth Innovation Promotion Association of the Chinese Academy of Sciences.
Article Link: https://www.cell.com/cell/fulltext/S0092-8674(23)00329-X#secsectitle0080
Contact: ZHANG Xinzheng
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: xzzhang@ibp.ac.cn
(Reported by Dr. ZHANG Xinzheng's group)

